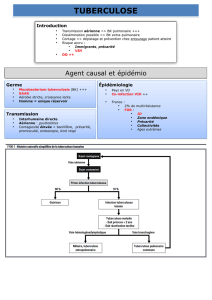
Angioedemes à kinine Nouvelles thérapeutiques en Europe

Angioedemes à kinine
Nouvelles thérapeutiques
en Europe
Pr David Launay
Service de Médecine Interne
Hôpital Claude-Huriez
CHRU Lille
CREAK : Centre National de Reference des Angioedemes à Kinine
C
Ré
Ak

Conflits d’intérêt avant la présentation
• Board Shire
• Board CSL Behring
• Grant de recherche SOBI
• Conseil pour ViroPharma

ACTUALITES THERAPEUTIQUES
• Actualités dans les traitements :
• nouveaux traitements
• développement en cours : clinicaltrials
• Actualités dans les stratégies
thérapeutiques : consensus et groupes de
travail nationaux et internationaux

Histoire de l’angioedeme héréditaire
1ere
description :
Graves
1840
oedeme
angioneurotique par
Quincke
Premier
article par
Osler
1882
1888
1963
Donaldson :
decouverte de
l’anomalie du C1
INH
Marasini et al. C1 INH
1976 et 1979 1985
pd C1INH (Berinert®)
icatibant (Firazyr®)
2008
nf C1INH (Cynrize®)
rhC1INH (Ruconest®)
pd C1INH (Berinert P®)

The new england journal of medicine
n engl j med 363;6 nejm.org august 5, 2010
582
by Zuraw and colleagues in this issue of the
Journal show the efficacy of nanofiltered C1 in-
hibitor concentrate.
5
In acute attacks, the dura-
tion of symptoms was significantly reduced by a
single intravenous injection, and the frequency
of attacks in severely affected patients was
halved by twice-weekly injections of nanofiltered
C1 inhibitor over a period of 12 weeks. Cinryze
received FDA approval for prophylaxis in heredi-
tary angioedema in 2008, but it has not yet been
approved for the treatment of acute attacks.
Concern about the transmission of viruses
also drove the development of recombinant C1
inhibitor (Rhucin, Pharming), which is expressed
in rabbits and harvested from their milk. Despite
half-life and immunogenicity issues, this agent
is currently being evaluated in clinical trials for
the treatment of acute attacks of hereditary angio-
edema. Now that the concern about the safety
of plasma C1 inhibitor has been largely resolved,
the need for an expensive recombinant protein
is questionable.
Safety concerns aside, C1 inhibitor is not an
ideal therapeutic agent. Lyophilized protein must
be stored properly, reconstituted carefully, and
administered intravenously, thus essentially re-
stricting its use to in-hospital treatment of acute
attacks. Administration at home, by either the
patient or a health care professional, is being
pilot-tested in some centers for both acute at-
tacks and prophylaxis, but the procedure is not
straightforward.
9
Alternative approaches are there-
fore needed.
Ecallantide (Kalbitor, Dyax) is a 60-amino-
acid protein selected from phage-display libraries
for its specific and potent inhibition of plasma
kallikrein; it has no effect on the complement
and coagulation systems. In early trials involving
more than 100 patients with hereditary angio-
edema, ecallantide had an acceptable side-effect
Coagulation cascade Complement cascade
Hereditary angioedema
Contact cascade
XII XIIa
XI
IX IXa (VIIIa)
XIa
C1 inhibitor
C1 inhibitor
Xa
Fibrinogen
Prothrombin Thrombin
Fibrin
X
Neutrophil influx
Endothelial leak Local pain
Inflammation Tissue edema
Increased clotting
C4
C4b2a
C2
C1r and C1s
MASP-2
C1 inhibitor
Complement
activation
C3a, C5a, and
membrane-attack complex
KallikreinPrekallikrein
C1 inhibitor
High-molecular-
weight kininogen
Bradykinin
Icatibant
Bradykinin type-2
receptors
Ecallantide
Endothelial-cell activation
1
Jarcho
7/08/10
AUTHOR PLEASE NOTE:
Figure has been redrawn and type has been reset
Please check carefully
Author
Fig #
Title
ME
DE
Artist
Issue date
COLOR FIGURE
Draft 3
Morgan
Knoper
8/05/10
Hereditary Angioedema:
Therapies Old and New
Figure 1. Dysregulation of Complement, Coagulation, and Contact Cascades in Hereditary Angioedema.
C1 inhibitor controls activation in the complement, coagulation, and contact cascades, and all three cascades are dysregulated in heredi-
tary angioedema. Replacement of C1 inhibitor restores homeostasis. Ecallantide and icatibant specifically inhibit the contact cascade
but have no direct effect on the complement or coagulation cascade. Dashed arrows indicate enzyme-cleavage steps, and T bars points
of inhibition. MASP-2 denotes mannose-binding lectin–associated serine protease 2.
The New England Journal of Medicine
Downloaded from nejm.org at INSERM DISC DOC on March 28, 2012. For personal use only. No other uses without permission.
Copyright © 2010 Massachusetts Medical Society. All rights reserved.
 6
6
 7
7
 8
8
 9
9
 10
10
 11
11
 12
12
 13
13
 14
14
 15
15
 16
16
 17
17
 18
18
 19
19
 20
20
 21
21
 22
22
 23
23
 24
24
 25
25
 26
26
 27
27
 28
28
 29
29
 30
30
 31
31
 32
32
 33
33
 34
34
 35
35
 36
36
 37
37
 38
38
 39
39
 40
40
 41
41
 42
42
 43
43
 44
44
 45
45
 46
46
 47
47
 48
48
 49
49
 50
50
 51
51
 52
52
 53
53
 54
54
 55
55
 56
56
 57
57
 58
58
 59
59
 60
60
 61
61
 62
62
 63
63
 64
64
 65
65
 66
66
 67
67
 68
68
 69
69
 70
70
1
/
70
100%