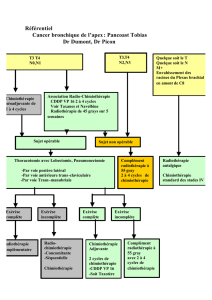
Séminome de stade I : les choix thérapeutiques : surveillance

Progrès en urologie (2011) 21, supplément 2, S53-S57
Séminome de stade I : les choix thérapeutiques :
surveillance, radiothérapie, chimiothérapie.
À propos d’un cas
Stage I seminoma: therapeutic strategy: surveillance, radiotherapy,
chemotherapy. A case-report
* Auteur correspondant.
Adresse e-mail : [email protected]
© 2011 Elsevier Masson SAS. Tous droits réservés.
Journées d’Onco-Urologie Médicale :
La pratique, les protocoles
25 et 26 juin 2010
67038
Volume 21 - Février 2011 - Supplément 1
ISSN 1166-7087
P. Bigot1,*
D’après les communications de S. Droupy2, A. Houlgatte3, R. De Crevoisier4
et A. Fléchon5
1 Service d’Urologie, CHU d’Angers, 4 rue Larrey, 49933 Angers CEDEX, France.
2 Service d’Urologie, CHU Carémeau, place Professeur Robert Debré,
30029 Nîmes CEDEX 9, France.
3 Service d’Urologie, hôpital Val-De-Grâce, 74 boulevard Port Royal,
75230 Paris CEDEX 05, France.
4 Département de Radiothérapie, Centre Eugène-Marquis, avenue Bataille Flandres
Dunkerque, 35042 Rennes CEDEX, France.
5 Département d’Oncologie Médicale, Centre Léon-Bérard, 28, rue Laennec,
69373 Lyon CEDEX 08, France.
Résumé
Les recommandations thérapeutiques dans la prise en charge des séminomes de stade I (pT1
à pT4, No, Mo) proposent la réalisation d’une surveillance, d’une chimiothérapie ou d’une
radiothérapie adjuvante. Ces différentes options thérapeutiques ne sont pas équivalentes
concernant la survenue d’effets secondaires et le risque de récidive. La surveillance est
indispensable car près de 15 % des patients sans traitement adjuvant présentent des récidives
tumorales. La survenue d’une récidive au cours d’une surveillance ne diminue cependant pas
la survie spécifi que. La radiothérapie lombo-aortique à la dose de 20 Gy permet de diminuer
le risque de rechute. Cette option a tendance à être moins utilisée en raison du risque à
long terme de cancer secondaire. La chimiothérapie par carboplatine permet également de
réduire le risque de récidive. Peu d’études évaluent à long terme les risques de rechute et
d’effets secondaires après chimiothérapie. Finalement, la décision thérapeutique doit être
partagée avec le malade après l’avoir informé de l’ensemble des possibilités thérapeutiques.
© 2011 Elsevier Masson SAS. Tous droits réservés.
MOTS CLÉS
Séminome
testiculaire ;
Radiothérapie ;
Surveillance ;
Chimiothérapie

S54 P. Bigot
Le cas clinique est le suivant
Un homme de 40 ans a subi une orchidectomie gauche après
la découverte par autopalpation d’une tumeur testiculaire.
Les marqueurs tumoraux pré-opératoires étaient un HCG à
10 UI, une alphafœtoprotéine normale et des LDH normaux.
Le scanner thoraco-abdominopelvien n’était pas en faveur de
localisations secondaires. En post-opératoire, le dosage de
l’HCG s’est normalisé et l’examen anatomopathologique a
révélé un séminome testiculaire pur de 4,5 cm de diamètre
associé à des emboles tumoraux vasculaires (pT2) et à une
infi ltration du rete testis.
Quelle prise en charge ?
Selon les recommandations de l’Association Européenne
d’Urologie (EAU) et de l’Association Française d’Urologie
(AFU), en cas de séminome testiculaire de stade I (pT1 à
pT4, N0, M0) les options thérapeutiques possibles sont [1-3] :
• la surveillance, qui comprend le dosage des marqueurs
et un scanner thoraco-abdominopelvien tous les 6 mois
pendant 5 ans ;
• la chimiothérapie adjuvante sous la forme d’une injection
de carboplatine AUC 7 ;
• la radiothérapie adjuvante para-aortique à une dose
totale de 20 Gy.
Vous exposez au patient toutes les options thérapeu-
tiques. Après vous avoir confi rmé qu’il avait parfaitement
compris que ses chances de guérison sont de 99 %, il vous
demande de l’orienter dans son choix. Quelle option théra-
peutique proposez-vous préférentiellement à ce patient ?
Quelle information donnez-vous au patient concernant les
risques immédiats et à long terme du traitement ?
La surveillance
Ce choix permet d’éviter tous les effets secondaires à court
et à long terme que l’on peut attribuer aux autres options
thérapeutiques, qu’il s’agisse de la radiothérapie ou de la
chimiothérapie. Il est néanmoins plus à risque de récidive
tumorale. Ainsi, au cours de la surveillance, les récidives
tumorales surviennent principalement la première année
et concernent 13 à 19 % des patients. Les récidives tardives
après la deuxième année sont rares et estimées de 1 à
3,6% des patients. La majorité des récidives se situent au
niveau des ganglions para-aortiques et leur traitement par
radiothérapie lomboaortique permet d’obtenir des résultats
équivalents à la radiothérapie de première intention sur la
survie spécifi que [4-6].
Les modalités de la surveillance ne sont pas similaires
entre les recommandations européennes et françaises. Les
guidelines 2010 de l’Association Européenne d’Urologie (EAU)
recommandent :
• la réalisation d’un dosage des marqueurs tumoraux
(βHCG, alphafœtoprotéine et LDH) et un examen clinique
tous les 4 mois les deux premières années, deux fois par
an la deuxième année puis une fois par an jusqu’à 5 ans ;
• une radiographie thoracique et un scanner thoraco-abdo-
minopelvien deux fois par an les deux premières années
puis une fois par an les trois années suivantes.
Le Comité de Cancérologie de l’Association Française
d’Urologie recommande [2,3,7] :
• la réalisation d’un dosage des marqueurs tumoraux (βHCG,
alphafœtoprotéine et LDH) et un examen clinique deux fois
par an pendant 5 ans puis une fois par an jusqu’à 10 ans ;
• un scanner thoraco-abdominopelvien deux fois par an
pendant 5 ans puis une fois par an jusqu’à 10 ans.
Ainsi, la surveillance proposée par les guidelines de
l’EAU recommande la réalisation de 7 scanners abdomino-
pelviens alors que celle proposée par le CCAFU recommande
15 scanners thoraco-abdominopelviens. Cependant, le
nombre et la nature des examens d’imagerie infl uent sur
le coût de la surveillance et l’irradiation induite. Il semble
ainsi nécessaire d’alléger et d’harmoniser les protocoles
de surveillance. Plusieurs études prospectives vont dans ce
sens et évaluent l’intérêt de l’IRM ou la suppression de la
radiographie pulmonaire [8,9]. Des auteurs ont également
proposé d’opter pour une surveillance uniquement en
KEYWORDS
Seminoma of the
testis;
Radiotherapy;
Chemotherapy;
Surveillance
Summary
The management guide-lines about stage I seminoma (pT1 à pT4, No, Mo) recommend
to perform a surveillance, an adjuvant chemotherapy based on carboplatine, or a
radiotherapy. However, these options are not equivalent for side effects and relapse
risk. Debates are in progress in order to simplify the surveillance protocols which remain
essential because of the tumoral relapses for 15% of the patients. The occurrence of a
tumoral relapse during the follow-up does not decrease the specifi c survival. The para-
aortic 20 Gy radiotherapy is effi cient on the seminoma and decreases the relapse risk. Its
main side-effect is a long-term risk of secondary cancer. Carboplatine chemotherapy is
also an effi cient option which provides good results on the specifi c survival and the survival
without progression. Very few studies assess the long-term side effects of chemotherapy.
In the end, the therapeutic decision must be taken with the patient after informing him
about all the therapeutic options.
© 2011 Elsevier Masson SAS. All rights reserved.

Séminome de stade I : les choix thérapeutiques : surveillance, radiothérapie, chimiothérapie. À propos d’un cas S55
Cette étude a inclus 1447 patients avec un suivi médian
de 4 ans. Les auteurs concluaient à une effi cacité similaire
des deux options thérapeutiques avec une survie spécifi que
à 5 ans de 96 % pour le groupe traité par radiothérapie et
de 94,5 % pour celui traité par chimiothérapie (Fig. 1).
Le taux de récidive des patients traités par radiothérapie
était de 4,7 % et 75 % des rechutes se situaient au niveau
lombo-aortique. Les effets secondaires précoces retrouvés
chez les patients traités par carboplatine étaient peu
nombreux avec principalement une asthénie modérée de
grade I-II (17 %) et plus rarement des toxicités neurolo-
giques de grade I (0,07 %), des nausées et vomissements
de grade III-IV (12,5 %) et des troubles hématologiques
à type de neutropénie (2 %) et thrombopénie (5 %) de
grade III-IV. Concernant la toxicité à long terme, peu de
données sont publiées sur la fertilité après chimiothérapie
par carboplatine et il est indispensable de réaliser une
conservation du sperme avant de débuter le traitement.
En revanche, le traitement adjuvant par carboplatine ne
semble pas provoquer de toxicité cardiovasculaire, rénale
ou de cancers secondaires à long terme. Enfi n, les patients
traités par chimiothérapie adjuvante conservent un risque
élevé de cancer sur le testicule controlatéral et le risque de
récidive après 5 ans n’est pas connu [23]. La surveillance
après chimiothérapie adjuvante est donc essentielle.
Cependant, il n’existe pas de recommandations précises
concernant ses modalités.
Conclusion
Les trois modalités thérapeutiques sont possibles chez
ce patient et la préservation de sa qualité de vie est un
objectif essentiel dans la mesure où sa guérison sera obtenue
dans plus de 95 % des cas. En cas de surveillance, le risque
de rechute sera plus important mais le risque de cancer
secondaire bien moindre. Radiothérapie et chimiothérapie
permettent de diminuer le risque de récidive au prix de
toxicités aiguës peu importantes mais de risques de cancers
secondaires plus élevés en cas de radiothérapie et de réci-
dives tardives non connues en cas de chimiothérapie. Dans
les trois confi gurations, les modalités de la surveillance après
traitement nécessitent d’être redéfi nies afi n de diminuer
l’absence ou en présence d’un seul facteur anatomopa-
thologique de mauvais pronostic (invasion du rete testis et
taille tumorale supérieure à 4 cm) [10].
Radiothérapie adjuvante
Le séminome est une tumeur très radiosensible et ce choix
permet de réduire le risque de récidive tumorale à 3 %. La
majorité des récidives (80 %) surviennent dans les 3 ans qui
suivent l’irradiation et sont localisées dans le pelvis. Après
récidive, les traitements par chimiothérapie conservent leurs
effi cacités [11].
Les modalités de la radiothérapie ont fortement évolué.
Ainsi le standard thérapeutique est aujourd’hui une irradiation
lombo-aortique conformationnelle de 20 Gy en 10 séances
et a une effi cacité comparable aux protocoles antérieurs
(irradiation lombo-aortique, iliaque, médiastinale et sus
claviculaire de 30 Gy) [12,13]. Cette évolution a permis de
limiter les toxicités. Les principales toxicités aiguës retrouvées
après radiothérapie sont digestives (nausées, vomissements,
diarrhées, gastrites) et les toxicités tardives « historiques » ont
quasiment disparu [14]. En effet, les grêles radiques étaient
liés à une irradiation de 30 Gy concentrée sur l’abdomen,
la toxicité cardiaque était consécutive à la radiothérapie
médiastinale et l’insuffi sance rénale était liée à l’irradiation
des reins qui sont à présent épargnés par la radiothérapie
conformationnelle. Cependant, l’hypofertilté secondaire à
la radiothérapie lombo-aortique atteint toujours 11 % des
patients et se traduit par une diminution de la paternité après
un traitement pour cancer du testicule. Ainsi Brydnoy et al.
ont rapporté respectivement 70 % et 92 % de paternité à
15 ans après radiothérapie adjuvante et surveillance [15].
Mais la principale problématique de la radiothérapie adjuvante
concerne le risque de second cancer. Ce risque est dépendant
du volume et de la distance après l’irradiation et correspond
à un risque relatif à 15 ans de second cancer multiplié par
2 (1,1-3,6). Les principales localisations tumorales secondaires
sont digestives, rénales, hématologiques et bronchiques. Ce
risque serait majoré chez les patients jeunes et après chimio-
thérapie [16,17]. Enfi n, il n’existe pas de recommandations
spécifi ques de surveillance après radiothérapie adjuvante et
classiquement ses modalités rejoignent celles éditées pour la
surveillance par l’EAU et le CCAFU.
Chimiothérapie adjuvante
Ce choix a pour objectif de diminuer le risque de rechute
tout en diminuant les toxicités à long terme par rapport à la
radiothérapie. De nombreuses études ont montré que cette
option était effi cace et permettait d’obtenir un taux de
rechute après traitement des séminomes de stade I allant
de 1,8 à 8,2 % (Tableau 1) [10,18-24]. Le standard théra-
peutique utilisé actuellement est un cycle de carboplatine
AUC 7 administré sur une période de 21 jours. Dans une
étude prospective de phase III randomisée, Oliver et al. ont
comparé les résultats, en termes de survie globale et de
récidive, de la chimiothérapie adjuvante par carboplatine
AUC 7 versus une irradiation lombo-aortique de 20 ou 30 Gy
chez des patients atteints d’un séminome de stade I [24].
Carboplatine
571 551 533 495 464 407 338 219 116 49 8
Survie sans récidive à 5 ans (IC95%)
Carboplatine : 94,7% ([92,5 ; 96,3])
Radiothérapie : 96,0% ([94,5 ; 97,1])
Survie sans récidive – Carboplatine vs Radiothérapie
Différence de survie sans récidive à 5 ans
Radiothérapie – Carboplatine : 1,34%
IC90% = [-0,7 ; 3,5]
Survie sans récidive
0
25%
75%
50%
100%
Radiothérapie
901 879 838 800 762 690 529 365 195 85 22
Figure 1. Survie spécifiques après radiothérapie adjuvante et
chimiothérapie adjuvante par un cycle de carboplatine AUC7 [24].

S56 P. Bigot
Tableau 1 Résultats des principales études de chimiothérapie adjuvante dans le séminome de stade I
Auteurs Année Nb.
de
patients
Dose
de carboplatine Nb.
de cycle Rechute Site
de la
rechute
Médiane
surveillance
Survie sans
récidive
à 5 ans
Survie
spécifi que
Oliver et al. 1994 78 450 mg/m201 : 25
02 : 53 0
1 (1,9 %) -
LA 29 mois
51 mois 100 %
98 % 100 %
100 %
Krege et al. 1997 43 400 mg/m2//21 j 2 0 LA 28 mois 100 % 100 %
Dieckmann et al. 2000 82 400 mg/m2//21 j 1 : 93
2 : 32 8 (8,6%)
0LA 48 mois
45 mois 91,10 % -
Reiter et al. 2001 107 400 mg/m2//21 j 2 0 - 74 mois 100 % 100 %
Steiner et al. 2002 108 400 mg/m2//21 j 2 2 (1,85 %) LA 60 mois 98,10 % 100 %
Aparicio et al. 2003 60 400 mg/m2//28 j 2 2 (3,3 %) - 52 mois 96,60 % 100 %
Argirovic et al. 2005 163 400 mg/m2//21 j 2 3 (1,9 %) LA 48 mois 98,90 % 100 %
Aparicio et al. 2005 131 AUC 7//21 j 2 7 (3,3 %) 6 LA
1 cordon 34 mois 96,2 % 100 %
Oliver et al. 2005 571 AUC 7//21 j 1 29 (5,2 %) LA 48 mois 94,8 % -
Powles et al. 2008 199
14 : 450 mg/m2
149 : AUC7
8 : AUC8
23 : 450 mg/m2
7 : AUC7
1
2
4(2 %)
0
2 LA
1 poumon
1 foie
108 mois 98 % 100 %
Swenoteca 2010 185 AUC7//21 j 1 8(4,2 %) - 56 mois 95,8 % 100 %
Aparicio 2010 74 AUC7//21 j 2 0 - 32 mois 100 % 100 %
LA : Lomboaortique

Séminome de stade I : les choix thérapeutiques : surveillance, radiothérapie, chimiothérapie. À propos d’un cas S57
[11] Martin JM, Panzarella T, Zwahlen DR, Chung P, Warde P.
Evidence-based guidelines for following stage 1 seminoma.
Cancer 2007;109:2248-56.
[12] Fossa SD, Horwich A, Russell JM, Roberts JT, Cullen MH, Hodson
NJ, et al. Optimal planning target volume for stage I testicular
seminoma: A Medical Research Council randomized trial.
Medical Research Council Testicular Tumor Working Group. J
Clin Oncol 1999;17:1146.
[13] Jones WG, Fossa SD, Mead GM, Roberts JT, Sokal M, Horwich A,
et al. Randomized trial of 30 versus 20 Gy in the adjuvant
treatment of stage I Testicular Seminoma: a report on
Medical Research Council Trial TE18, European Organisation
for the Research and Treatment of Cancer Trial 30942
(ISRCTN18525328). J Clin Oncol 2005;23:1200-8.
[14] Aass N, Fossa SD, Host H. Acute and subacute side effects due
to infra-diaphragmatic radiotherapy for testicular cancer: a
prospective study. Inter J Rad Oncol Biol Phys 1992;22:1057-64.
[15] Brydoy M, Fossa SD, Klepp O, Bremnes RM, Wist EA, Wentzel-
Larsen T, et al. Paternity following treatment for testicular
cancer. J Natl Cancer Inst 2005;97:1580-8.
[16] Bachaud JM, Berthier F, Soulie M, Malavaud B, Plante P,
Rishmann P, Chevreau C, Grosclaude P. Risque de deuxième
cancer non germinal après traitement d’un séminome testi-
culaire de stade I-II. Prog Urol 1999;9:689-95.
[17] Travis LB, Fossa SD, Schonfeld SJ, McMaster ML, Lynch CF,
Storm H, et al. Second cancers among 40,576 testicular
cancer patients:focus on long-term survivors. J Natl Cancer
Inst 2005;97:1354-65.
[18] Oliver T. One-dose carboplatin in seminoma. Lancet 2005;366:
1526.
[19] Krege S, Kalund G, Otto T, Goepel M, Rubben H. Phase II study:
adjuvant single-agent carboplatin therapy for clinical stage I
seminoma. Eur Urol 1997;31:405-7.
[20] Reiter WJ, Brodowicz T, Alavi S, Zielinski ML, Kozak W, Maier U,
et al. Twelve-year experience with two courses of adjuvant
single-agent carboplatin therapy for clinical stage I seminoma.
J Clin Oncol 2001;19:101-4.
[21] Argirovic D. Germ cell testicular tumors in clinical stage A and
normal values of serum tumor mark-ers post-orchiectomy: the
experience in the management of 300 consecutive patients. J
Buon 2005;10:195-200.
[22] Steiner H, Holtl L, Wirtenberger W, Berger AP, Bartsch G,
Hobisch A. Long-term experience with carboplatin monothe-
rapy for clinical stage I seminoma: a retrospective single-center
study. Urology 2002;60:324-8.
[23] Powles T, Robinson D, Shamash J, Moller H, Tranter N, Oliver T.
The long-term risks of adjuvant carboplatin treatment for stage
I seminoma of the testis. Ann Oncol 2008;19:443-7.
[24] Oliver RT, Mason MD, Mead GM, von der Maase H, Rustin GJ,
Joffe JK, et al. Radiotherapy versus single-dose carboplatin in
adjuvant treatment of stage I seminoma: a randomised trial.
Lancet 2005;366:293-300.
l’irradiation secondaire aux examens d’imagerie tout en
conservant l’opportunité de dépister les récidives à un stade
précoce. Dans cette situation, l’information au patient des
différentes modalités thérapeutiques, de leurs avantages
et de leurs inconvénients est donc primordiale car ce sont
principalement ses exigences qui guideront le choix entre
surveillance, radiothérapie et chimiothérapie adjuvantes.
Déclaration d’intérêts
Aucune.
Références
[1] Kreger S, Beyer J, Souchon R, Albers P, Albrecht W, Algaba
F, et al. European consensus conference on diagnosis and
treatment of germ cell cancer:a report of the second
meeting of the European Germ Cell Cancer Consensus group
(EGCCCG):part I. Eur Urol 2007;53:478-96.
[2] Mottet N, Culine S, Iborra F, Avances C, Bastide C, Lesourd A,
et al. Tumeurs du testicule. Prog Urol 2007;17:1035-45.
[3] Durand X, Rigaud J, Avances C, Camparo P, Culine S, Iborra
F, Mottet N, Sèbe P, Soulié M. Recommandations en Onco-
Urologie 2010 : Tumeurs germinales du testicule. Prog Urol
2010;20:S297-S309.
[4] Francis R, Bower M, Brunstrom G, Holden L, Newlands ES,
Rustin GJ, et al. Surveillance for stage I testicular germ cell
tumours: results and cost benefi t analysis of management
options. Eur J Cancer 2000;36:1925-32.
[5] Chung P, Parker C, Panzarella T, Gospodarowicz MK, Jewett S,
Milosevic MF, et al. Surveillance in stage I testicular seminoma
– risk of late relapse. Can J Urol 2002;9:1637-40.
[6] Cummins S, Yau T, Huddart R, Dearnaley D, Horwich A.
Surveillance in Stage I Seminoma Patients: A Long-Term
Assessment. Eur Urol 2010;57:673-8.
[7] Culine S, Michel F, Rocher L, Mottet N, Davin JL. Suivi des
tumeurs germinales du testicule. Recommandations du Comité
de Cancérologie de l’Association Française d’Urologie. Prog
Urol 2005;15:593-6.
[8] Wood L, Kollmannsberger C, Jewett M, Chung P, Hotte S, O’Malley
M, et al. Canadian consensus guidelines for the management of
testicular germ cell cancer. Can Urol Assoc J 2010:4:e19-38
[9] Tolan S, Vesprini D, Jewett MA, Warde PR, O’Malley M,
Panzarella T, et al. No role for routine chest radiography in
stage I seminoma surveillance. Eur Urol 2010;57:474-9.
[10] Aparicio J, Robert L, Muro XG, Guma J, Sanchez A, Margeli M,
et al. Risk-adapted management of stage I seminoma: Results
of the third Spanish Germ Cell Cancer Group study. J Clin Oncol
2010;28:15s: Abstract 4532.
1
/
5
100%