ECOLE DOCTORALE "Médicament, Toxicologie, Chimie, Imageries

ECOLE DOCTORALE "Médicament, Toxicologie, Chimie, Imageries"
UNIVERSITE SORBONNE PARIS CITE
Proposition de sujet de thèse à l’appui d’une demande de contrat doctoral 2016-2017
Version word sans signature pour le site et version papier signée.
Renseignements relatifs à l’Unité de Recherche :
Nom et appartenance : UMR3666
Nom et prénom du Directeur : Ludger Johannes
Téléphone :0622525051
courriel : ludger.johanne[email protected]
Signature obligatoire :(et avis éventuel) : Très Favorable
Renseignements relatifs à l’Equipe d’Accueil (EAD) :
Nom de l’Equipe d’Accueil : Chemical Cell Biology Team
Nom et prénom du responsable : Raphaël Rodriguez
Qualité du responsable : Chargé de Recherche CR1, Chef d’équipe Institut Curie
Téléphone :0648482191
courriel : raphael.rodriguez@curie.fr
Signature obligatoire:
Renseignements relatifs au sujet de thèse :
Nom et prénom du Directeur de thèse (HDR) : Raphaël Rodriguez
Qualité : Chargé de Recherche CR1, Chef d’équipe Institut Curie
Téléphone :0648482191
courriel : raphael.rodriguez@curie.fr
Signature obligatoire :
Titre du sujet proposé : Targeting iron metabolism in cancer stem cells
Mots clés disciplinaires * : Cancer, fer, chemobiologie
Nombre de thèses encadrées ou co-encadrées : 11
Préciser le secteur disciplinaire (402, 510, 530)* principal et éventuellement secondaire : 402
Résumé succinct (5 lignes maximum) :
This research project seeks to design and synthesize a series of small molecules derived from the
complex natural product salinomycin with the view to selectively target iron metabolism in cancer
stem cells. To this end, we will take advantage of previous expertise in this research area that we
have acquired over the years. In a second time, we aim to use these small molecules to delineate
the role of iron in the maintenance of cancer stem cells.
Demande dans le cadre d’un projet ANR (donner toutes précisions utiles) : Non éligible

ECOLE DOCTORALE "Médicament, Toxicologie, Chimie, Imageries"
- UNIVERSITE PARIS DESCARTES
Proposition de sujet de thèse à l’appui d’une demande de contrat doctoral 2016-
2017
(l'ensemble de cette fiche ne doit pas dépasser 1 page)
Nom, prénom du directeur de l'unité de recherche :Ludger Johannes
Numéro de l'unité de recherche (et établissement de rattachement) : UMR 3666
Nom, prénom du responsable de l'équipe d'accueil (EAD) : Raphaël Rodriguez
Nom, prénom du directeur de thèse : Raphaël Rodriguez
Titre du sujet de thèse proposé : Targeting iron metabolism in cancer stem cells
Contenu scientifique du programme de la thèse
We aim to develop drugs that selectively target cancer stem cells (CSCs), the main cause of drug
resistance and cancer relapse, for which there is currently no clinically approved treatment. We have
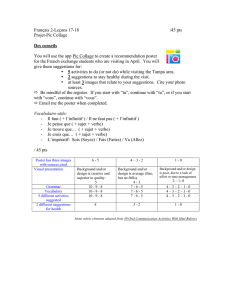
previously shown that the natural product salinomycin (Figure 1) and its synthetic derivative ironmycin
do not operate as ionophores but target instead the lysosomal compartment and interact with iron(II),
thereby preventing release of the metal into the cytosol. This in turns leads to cellular iron depletion
and the production of lethal reactive oxygen species in this organelle. Our study revealed that iron
homeostasis is upregulated in cancer stem cells and represents a druggable network.
In the first part of this project, we will develop new drugs based on our prototype drug ironomycin
that already exhibits a 10-fold increased potency compared to salinomycin with improved selectivity
CSCs vs normal cancer cells. In the second part of this project, we aim to elucidate the role of iron in
the maintenance of CSCs. We have recently found (unpublished results) that endothelial-to-
mesenchymal transition (EMT) in cancer models requires iron. Thus, we hypothesized that the
epigenetic reprogramming that promote EMT is mediated by iron-dependent enzymes. In particular,
TET and Jumonji enzymes have previously been shown to regulate the epigenetic landscape through
iron-mediated oxidative demethylation processes. This project will combine state-of-the-art
chemistry and cell biology.
Figure 1: Molecular structure of salinomycin (upper panel) and ironomycin (lower panel)
Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Trang Thi Mai, Ahmed
Hamaï, Antje Hienzsch, Tatiana Cañeque, Sebastian Müller, Julien Wicinski, Olivier Cabaud,
O
O
O
O
O
OH
OOH
OH O
O
O
O
O
O
O
OH
OOH
OH O
NH
O
O
O
O
O
OH
OOH
OH O
OH
O
O
O
O
O
O
OOH
OH O
NH
H
H
H
H
H H
H
H
H
H
H
HH
H

Christine Leroy, Amandine David, Verónica Acevedo, Akihide Ryo, Christophe Ginestier,
Daniel Birnbaum, Emmanuelle Charafe-Jauffret, Patrice Codogno, Maryam Mehrpour,
Raphaël Rodriguez* Nature Chem. In Press
1
/
3
100%