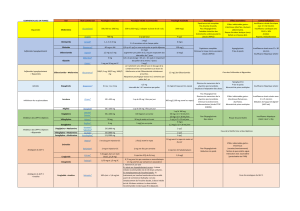
tableau récapitulatif des anti

DCI Nom'commercial Posologies'existantes Posologie'initiale'recommandée Posologie'maximale Avantages Effets'secondaires Contre-indications
!"#$%&'()*+ ,--./0,-/%#/1---/2) ,--/%#/0,-/2)34./5/(6(&7*8/(#/9%#7/6*/1-/5/1,4 :---/2)34
;7()<6+ =--/2) :/$&/&(8/4%#8 >/$&34
Glimépiride ?2(8*"+ 1./@./:/*7/>/2)
1/2)34
1/5/@/A*2(<B*A/*B78*/$'(C#*/&("<*8
D/2)34
Gliclazide E<(2<$8%B+ D-/2)/*B/FG -.,/$&/5/@/$&34/*B/#B*/A*#"*/&8<A*/(#/&*7<7H6I4*#B*8 1@-/2)34
Glibenclamide E(%B<"+ @.,/2)/%#/,/2)
@.,/2)34
(#)2*B7*8/&(8/&("<*8/6*/@.,/2)/*B/8*A&*$7(B7/6*A/
<B7*8J(""*A/6*/=/4%#8A
1,/2)34
Glipizide
!"<9IBKA*+
LM<6<(+
,/2)
,/2)/%#/1-/2)/*B/FN
-.,/5/>/$&34/OA</P)*/Q/D,/(BA/R/-.,/$&34/*B/8*A&*$7(B7/
6*A/&("<*8A/6S(#/2%<BA/=/4%#8AT
,/2)34
!"#$%&'()*+,-./0#-12&'%34*+
5+6'0"%3'()*
Glibenclamide'+'Metformine !"#$%J(B$*+
,--3@.,/2)./,--3,/2)./1---3,/
2)
F*/78(<7*2*B7/A*8(/6I9#7I/(J*$/"*/6%A()*/6*/"(/
$%29<B(<A%B/U<V*/$%88*A&%B6(B7/(#V/6%A*A/6*/
G*7U%82<B*/*7/6*/!"<9*B$"(2<6*/<B<7<("*2*B7/
&8*A$8<7*AW
?6(&7(7<%B/&%A%"%)<C#*/7%#7*A/"*A/@/A*2(<B*A/&(8/
&("<*8/6*/1/$&
1,/2)34/6*/!"<9*B$"(2<6*
7#'3'()* Répaglinide X%J%B%82+
-.,/%#/1/%#/@/2)
-.,/2)
<B7*8J(""*/6*/1/5/@/A*2(<B*A/&(8/&("<*8 1D/2)34/O>/2)/(J(B7/"*A/8*&(AT
E<2<B#*/"*A/*V$#8A<%BA/6*/"(/
)"Y$I2<*/&%A7H&8(B6<("*W
;%#&"*AA*/6S(6(&7(7<%B/6*A/6%A*A
ZY&%)"Y$I2<*
N8<A*/6*/&%<6A
XI$*AA<7I/6*/&8<A*A/2#"7<&"*A
[BA#UU<A(B$*/'I&(7<C#*/AIJK8*
Acarbose !"#$%8+ ,-/5/1--/2) 1/$&/6*/,-/2)/:/U%<A/&(8/4%#8
Miglitol E<(A7(9%"+ ,-/5/1--/2) 1/$&/6*/,-/2)/:/U%<A/&(8/4%#8
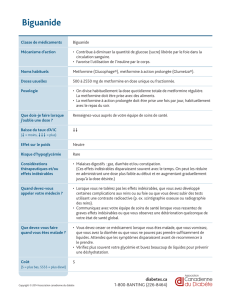
Sitagliptine
\(B#J<(+
]*"*J<(+
,-/5/1--/2) 1--/2)34/*B/#B*/&8<A*
1/$&3/5/1--/2)
,-/2)/A</^"/_/,-/2F32<B
@,/2)/A</^"/_/:-/2F32<B
Vildagliptine !("J#A+ ,-/2) ,-/2)/"*/2(7<B/*7/"*/A%<8 1/$&34/A</^"/_/,-/2F32<B
Saxagliptine LB)"YM(+ ,/2) ,/2)34/*B/#B*/&8<A*
,/2)34
@.,/2)34/A</^"/_/:-/2F32<B
Linagliptine `8(4*B7(+
,/2)
,/2)34
Alogliptine a<&<6<(+ D.@,/2)./1@.,/2)./*7/@,/2) @,/2)34
Sitagliptine'+'Metformine
\(B#2*7+
a*"2*7<(+
,-31---/2) @/$&34
Vildagliptine'+'Metformine b#$8*(A+ ,-31---/2) @/$&34
Saxagliptine'+'Metformine c%29%)"YM*+ @.,31---/2) @/$&34
Alogliptine'+'Metformine a<&6%2*7+ ,-31---/2)
Linagliptine'+'Metformine \*B7(6#*7%+ @.,31---/2)
Exénatide
dY*77(+
dY6#8I%B+
,/*7/1-/e)/*B/<B4*$7<%B/;^
@/2)/*B/<B4*$7<%B/;^
@/U%<A34/(J(B7/"*/8*&(A
/@/2)/#B*/U%<A/&(8/A*2(<B*
1-/e)/(J(B7/"*/8*&(A/6#/2(7<B/*7/
6#/A%<8
1/<B4*$7<%B/;^/'*96%2(6(<8*
Liraglutide a<$7%M(+
:/6%A()*A/6(BA/#B/A7Y"%/
1/<B4*$7<%B/;^34/6*/-.D/2) 1.0/2)34
Dulaglutide `8#"<$<7Y+
-.=,/2)/*7/1.,/2)/;^
-.=,/2)/#B*/U%<A/&(8/A*2(<B*/*B/2%B%7'I8(&<*
1.,/2)/#B*/U%<A/&(8/A*2(<B*/*B/(AA%$<(7<%B
Lixisenatide FYV#2<(+ 1-/e)/*7/@-/e) 1-/e)/#B*/U%<A/&(8/4%#8/&*B6(B7/1>/4%#8A/&#<A/@-/e) @-/e)34
83%#/0")*+("+79:;<+
5+=3*"#'3)
Liraglutide'+'insuline ]#"7%&'Y+ 1--/#32F/f/:.D/2)32F
1/<B4*$7<%B/;^/&(8/4%#8
bB/(4%#7/(#V/'Y&%)"Y$I2<(B7A/%8(#V/R/"(/6%A*/
<B<7<("*/8*$%22(B6I*/*A7/6*/1-/6%A*A/#B<7(<8*AW
bB/8*2&"($*2*B7/6*/"S<BA#"<B*/9(A("*/R/"*/
78(<7*2*B7/&(8/<BA#"<B*/9(A("*/6*J8(/g78*/(88g7I/
(J(B7/6*/$%22*B$*8/]#"7%&'YW/F%8A/6#/
8*2&"($*2*B7/6*/"S<BA#"<B*/9(A("*./"(/6%A*/<B<7<("*/
*A7/6*/1D/6%A*A/#B<7(<8*AW/F(/6%A*/<B<7<("*/
8*$%22(B6I*/B*/6*J8(/&(A/g78*/6I&(AAI*W/
Dapagliflozine h%8V<)(+ 1-/2) 1-/2)34 1-/2)34
Canagliflozine [BJ%i(B(+ 1--/2)/*7/:--/2) 1--/2)/#B*/U%<A/&(8/4%#8 :--/2)34//
Empagliflozine \(86<(B$*+ 1-/2)/*7/@,/2) 1-/2)/#B*/U%<A/&(8/4%#8 @,/2)34
Dapagliflozine'+'Metformine ]<)6#%+ ,31---/2) 1/$&/@/U%<A/&(8/4%#8 1-3@---/2)
Canagliflozine'+'Metformine a%i(B(2*7+ ,-31---/2)/*7/1,-31---/2) ,-31---/2)/@/U%<A/&(8/4%#8 :--3@---/2)
bV&I8<*B$*/78KA/$%2&"K7*W/
N(A/6*/&8<A*/6*/&%<6AW/
N(A/6S'Y&%)"Y$I2<*W/
N8%9(9"*/8I6#$7<%B/6*A/
IJKB*2*B7A/$(86<%J(A$#"(<8*A/
OI7#6*/jcNE;T
bUU*7A/<B6IA<8(9"*A/)(A78%H
<B7*A7<B(#V/O6<(88'I*./6%#"*#8A/
(96%2<B("*ATW/
k<AC#*/6S($<6%A*/"($7<C#*/O8(8*TW/
EIU<$<7/*B/a<7(2<B*/d1@W
[BA#UU<A(B$*/8IB("*/$'8%B<C#*/
(J*$/^"/_/D-/2F32<B/
[BA#UU<A(B$*/$(86<(C#*/(J*$/
hba!/_/:-l/
?$<6%A*/
ZY&%V<*./EIA'Y68(7(7<%B
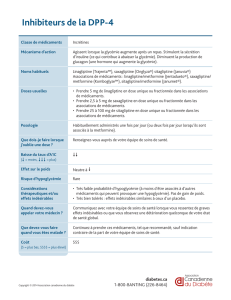
6'0"%3'()*
Metformine
N(A/6S'Y&%)"Y$I2<*
d<*B/7%"I8I
=3,'>'4)"?*+()*+@::A+B+7#'.4'3)*
k<AC#*/6*/&(B$8I(7<7*
[BA#UU<A(B$*/'I&(7<C#*/
O?;?`./?F?`/Q/:XT
bV&I8<*B$*/$%2&"K7*W
E<2<B#*/"*/8<AC#*/2<$8%HJ(A$#"(<8*/
OI7#6*/jcNE;T
ZY&%)"Y$I2<*
N8<A*/6*/&%<6A
[BA#UU<A(B$*/8IB("*/(J*$/^"/_/:-/
2F32<B
[BA#UU<A(B$*/'I&(7<C#*/AIJK8*
!"#$%&'()*+,-./0#-12&'%34*
:--/2)34/O1--/2)/(J(B7/"*A/8*&(AT
N(A/6S'Y&%)"Y$I2<*
E<2<B#*/"*A/*V$#8A<%BA/6*/"(/
)"Y$I2<*/&%A7H&8(B6<("*W
E<2<B#*/"*A/IJKB*2*B7A/
$(86<%J(A$#"(<8*A/OI7#6*/;`LN/
X[EEGT
bUU*7A/<B6IA<8(9"*A/)(A78%H
<B7*A7<B(#V/OU"(7#"*B$*A./
6<(88'I*ATW
XI$*AA<7I/6*/&8<A*A/2#"7<&"*A
=3,'>'4)"?*+()*+
⍺
;0#"1/*'(%*)*
[BA#UU<A(B$*/8IB("*/AIJK8*/(J*$/
^"/_/@,/2F32<B
G("(6<*A/6*/"S(&&(8*<"/6<)*A7<U/
OG[^[/*7$T
^*#V/6*/"(/G*7U%82<B*/*7/6*A/[B'<9<7*#8A/6*A/;`F)@
=3,'>'4)"?*+()*+!C90D+5+6'0"%3'()*
^*#V/6*A/;#"U(2<6*A/*7/d<)#(B<6*A
^*#V/6*A/(B("%)#*A/6#/!FNH1
[BU*$7<%BA/#8<B(<8*A
GY$%A*A/)IB<7("*A
ZY&%J%"I2<*/$'*M/"*/A#4*7/P)I
=3,'>'4)"?*+()*+!C90D
[BA#UU<A(B$*/8IB("*/
(J*$/^"/_/D-/2F32<B
^*#V/6*/"(/G*7U%82<B*/*7/6*A/!"<&7<B*A
=3,'>'4)"?*+()*+@::A+B+7#'.4'3)*+
5+6'0"%3'()*
N(A/6S'Y&%)"Y$I2<*
kI6#$7<%B/6#/&%<6A
bUU*7A/<B6IA<8(9"*A/)(A78%H
<B7*A7<B(#V/
OB(#AI*A3J%2<AA*2*B7AT
X%7<%B/6*/&(B$8I(7<7*/(<)#m
[B7*8($7<%B/(J*$/^%#2(6<B*/
O&*87#89(7<%B/6*/"S[XkT
83%#/0")*+("+79:;<
1
/
1
100%