Cours Boussaoud Partie 02.pptx

22/11/15
1
!"#$%&$'()&%*+%&$'(+,,-#$%..+/#(
012+3-+/#(4#(2+(-#15#-15#(6&$4+)#$7+2#(
Driss%Boussaoud!
"#$%&'%($!)%!*%&+%$&+%!,(!-.*/!
3. Apprentissage par renforcement
! Historique sur le renforcement
! Conditionnement, apprentissage
! Apprentissage associatif, basé sur le
renforcement
! Apprentissage par imagerie mentale
! Apprentissage social
Edward L. Thorndike
Williamsburg, Mass.
1874 - New York 1949
Apprentissage, conditionnement
The law of effect
• 0%!1$%2#%$!134&+5657(%!8!9'()#%$!6%!
&5:)#'#5::%2%:'!519$,:';!
• /5:!%<19$#%:&%!6,!16(3!&96=>$%!,?%&!
)%3!&+,'3!16,&93!),:3!6,!@>5#'%A
1$5>6=2%B;!
Thorndike, E. L. (1898). Animal intelligence.
La «!boite-problème!» de Thorndike
• C+5$:)#D%!&5:&6('!89#(
7&97(1&),&-7#)#$7(
893(+(:7:(.93*3(4;9$#(
1&$.:89#$1#(,2+3.+$7#(
7#$4(<(=7-#(-#,-&4937'(
#7(3$*#-.#)#$7(7&97(
1&),&-7#)#$7(.93*3(
4;9$#(1&$.:89#$1#(
4:,2+3.+$7#(7#$4(<(=7-#(
3$53>:?(2&3(4#(2;#@#7(
A2+B(&6(#@#17C(

22/11/15
2
Deux formes d’effet : renforcement et
punition, bases du conditionnement
E53#'#F!
.97,'#F!
*%:F5$&%2%:'!
0,!1$5>,>#6#'9!)%!$915:3%!
,(72%:'%!
E(:#'#5:!
0,!1$5>,>#6#'9!)%!$915:3%!
)#2#:(%!
*9&521%:3%!/'#2(6(3!,?%$3#F!
,116#G(9(
/'#2(6(3!,?%$3#F!!
$%'#$9!
*9&521%:3%!
$%'#$9%!(
H($$+(3!I$%)%$#&!/JK..L*!
Susquehanna, Penn. 1904,
Cambridge, Mass. 1990
light
bar
food tray
water
-,7%!)%!/D#::%$!
Conditionnement classique versus opérant
*9&521%:3%!)#31%:39%!#:)91%:),22%:'!)(!&5215$'%2%:'!)(!$,'!
0%!&5215$'%2%:'!)(!$,'!)9'%$2#:%!6,!$9&521%:3%!
-6,33#G(%!!
*9M%<%
N19$,:'!!
O565:',#$%
De l’apprentissage à la mémoire
• 0%!&5:)#'#5::%2%:'!&5:)(#'!8!6,!2925$#3,'#5:!
)%3!$%6,'#5:3!%:'$%!3'#2(6#!5(!%:'$%!3'#2(6#!%'!
&5215$'%2%:'3P!
• 0%!&5:)#'#5::%2%:'!519$,:'!&5:)(#'!8!6,!
F5$2,'#5:!)%!6,!2925#$%!1$5&9)($,6%Q!5(!6,!
2925#$%!)%3!+,>#'()%3!R+,>#'!2%25$4SP!
• T:%!2925#$%!8!65:7!'%$2%Q!)5:'!6%!3(115$'!
:%($5:,6!%3'!6%!&5$'%<!F$5:',6Q!6%3!7,:76#5:3!)%!6,!
>,3%!%'!6%3!3'$(&'($%3!)(!65>%!'%215$,6!29)#,:P!

22/11/15
3
Les différents types de mémoire
U925#$%!
V!&5($'!'%$2%!
@!!U925#$%!)%!'$,?,#6!B!V!65:7!'%$2%!
U925#$%!)%3!F,#'3!
%'!)%3!9?9:%2%:'3!
U925#$%!)%3!
3,?5#$AF,#$%!
-5$'%<!1$9F$5:',6!
/43'=2%!6#2>#G(%!
/43'=2%!F$5:'5A3'$#,',6!
%'!&%$?%6%'!;;;!
W,>#6'93!25'$#&%3!+(2,#:%3!
0%!&5:&%1'!)X,11$%:'#33,7%!
" !0X,11$%:'#33,7%!%3'!(:!&+,:7%2%:'!)(!&5215$'%2%:'!G(#!
$93(6'%!)%!6X%<19$#%:&%!1,339%P!!
" !0,!&,1,&#'9!)X,11$%:)$%!%<#3'%!&+%Y!6%3!%319&%3!,:#2,6%3!
R,:#2,6!6%,$:#:7!'+%5$4S!%'!1%('!Z'$%!1$57$,229%!),:3!)%3!
2,&+#:%3!R2,&+#:%!6%,$:#:7SP!
" !0,!1$%:'#33,7%!?#3%!8!$9)(#$%!6X9&,$'!%:'$%!6,!&5:39G(%:&%!
,''%:)(%!)X(:!&5215$'%2%:'!%'!6,!&5:39G(%:&%!1$5)(#'%P!
" !K6!$93(6'%!)%!6,!16,3'#&#'9!:%($5:,6%Q!5(!:%($516,3'#&#'9;!
0,!:%($516,3'#&#'9!
" !L66%!%<#3'%!2Z2%!),:3!6%!&%$?%,(!
,)(6'%[!
0%3!:%($5:%3!1%(?%:'!25)#\%$!6%($3!
1$51$#9'93!#:)#?#)(%66%3!%'!6%($3!
#:'%$,&'#5:3Q!1,$!6%!>#,#3!)%!
25)#\&,'#5:3!34:,1'#G(%3!R65#!)%!
W%>>S!
-%3!25)#\&,'#5:3!1%$2%''%:'!
)X,11$%:)$%!%'!)%!F5$2%$!)%!
:5(?%,(<!35(?%:#$3Q!2,#3!,(33#!6,!
$9&(19$,'#5:!F5:&'#5::%66%;!
W%>>X3!6,][!^:%($5:3!
'+,'!\$%!'57%'+%$Q!
]#$%!'57%'+%$_!'+%4!
F5$2!&%66!,33%2>6#%3`

22/11/15
4
0X,11$%:'#33,7%Q!(:!&+,21!2(6'#)#3#:,#$%!
" !.%($53&#%:&%3Q!3&#%:&%3!
&57:#'#?%3Q!134&+5657#%Q!29)%&#:%!
)%!6,!$9+,>#6#','#5:Q!3&#%:&%3!)%!
6X9)(&,'#5:_!
" !0X#:'%66#7%:&%!,$'#\&#%66%Q!6,!
$5>5'#G(%Q!3&#%:&%3!)%!6X#:79:#%($Q!
6X#:F5$2,'#G(%Q!2,'+92,'#G(%3!%'!
1+43#G(%P!
" K6!%3'!>,39!3($!6%!$%:F5$&%2%:';!
E$#:,(<!'41%3!)X,11$%:'#33,7%!
L<19$#%:&%!#:)#?#)(%66%! V11$%:'#33,7%!35&#,6!
V11$%:'#33,7%!1,$!
#2,7%$#%!2%:',6%!
S R O
Stimulus Réponse Conséquence
-#$&(#'3!)%!6X,11$%:'#33,7%!1,$!$%:F5$&%2%:'!
a,:76#5:3!
)%!6,!>,3%!
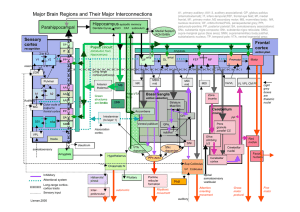
-5$'%<!I$5:',6!
D&-7#E(
F+.+2(
/+$/23+(
Thalamus
Cortico-basal
ganglia loops
+
+
-
G#9-&,2+.%137:(H(13-1937.(4#(2+(4&,+)3$#(
• I:1&),#$.#JK2+3.3-(
• D&4+/#(4#(2;#--#9-(
• L&%*+%&$JM:13.3&$(
GPe
STN
+
-
-
GPi/SNr
Thalamus
+
-
-
Striatum
Cortex
SNc
Dopamine
+/-
+/-
+ + +

22/11/15
5
N O+3-#(49(*:2&(
N D&4#(4#(2+(-&97#(
N P#179-#(QQQ(
(
Neuroplasticité & circuits de la
dopamine !,,-#$%..+/#(+..&13+%6(
• 0,!)9&+,$7%!)%3!:%($5:%3!
)51,2#:%$7#G(%3!)9&+,$7%:'!
)X,(',:'!16(3!F5$'%2%:'!G(%!
6X%$$%($!)%!1$9)#&'#5:!%3'!
7$,:)%P!
• K63!1$5b%''%:'!3($!6%!3'$#,'(2!%'!6%!
&5$'%<!F$5:',6Q!)5:'!#63!25)#\%:'!
6X,&'#?#'9!15($!,),1'%$!6%!
&5215$'%2%:'Q!)%!35$'%!8!
$9)(#$%!6X%$$%($!)%!1$9)#&'#5:;!
Dopamine codage de l’erreur de prediction
!"#$$#%$&'#&($)'*+,*-.&#/,&0")+1$,&#.,$#&01&+-./)2%#.+#&1,,#.'%#&#,&01&
+-./)2%#.+#&($-'%*,#&(1$&0#&+-3(-$,#3#.,4&
Wolfram Schultz and Anthony Dickinson (2000). NEURONAL CODING OF
PREDICTION ERRORS. Annu. Rev. Neurosci. 2000. 23:473–500.
"51,2#:%!25)#\%3!34:,1'#&!%c&,&4!RW%>>S!
#:!F$5:',6!&5$'%<!,:)!>,3,6!7,:76#,!A!U%25$4!
DA
A retenir:
• 0%3!:%($5:%3!)51,2#:%$7#G(%3!b5(%:'!(:!$d6%!
&%:'$,6!),:3!6X,11$%:'#33,7%!1,$!
$%:F5$&%2%:'P!
• K63!)9&+,$7%:'!%:!$%6,'#5:!,?%&!6,!$9&521%:3%!
R$%:F5$&%2%:'S!%'!'5('!9?9:%2%:'!)%!
6X%:?#$5::%2%:'!G(#!6,!1$9)#'!R25'#?,'#5:SP!
• 0,!"V!25)#\%!6,!'$,:32#33#5:!34:,1'#G(%!
),:3!6%3!$97#5:3!&#>6%3!R&5$'%<!F$5:',6!&5$'%<!
%'!7,:76#5:3!)%!6,!>,3%S!!!2925$#3,'#5:;!
 6
6
 7
7
 8
8
 9
9
 10
10
1
/
10
100%