V-39 Facteurs de croissance hématopoïétiques

V"#39#FACTEURS#DE#CROISSANCE#HEMATOPOIETIQUES#–#CYTOKINES#ET#ANTAGONISTES#
!
!
"#$%&$'()*'+!
,!
Facteurs#de#croissance#hématopoïétiques#
#
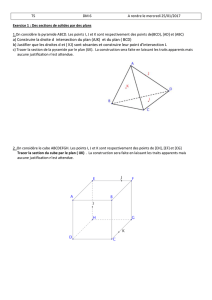
Introduction#:#l’hématopoïèse#
! !
"#$%&'()*)+,-.!/)0-1-(.!.0!2'!*3)214%3'(1)0!.(!2'!&'(53'(1)0!6.-!/.2252.-!-)5/$.-!*)53!
)7(.013!6.-!/.2252.-!-'08510.-!4)0/(1)00.22.-!.(!3.*3%-.0('(19.-!6.-!6144%3.0(.-!2180%.-:!
!;.5<!/'3'/(%31-(1=5.-!%9)25.0(!.0!-.0-!109.3-.!>!
? 2'!*)2@9'2.0/.!)5!*2531*)(.0/.!>!/'*'/1(%!6.!-.!6144%3.0/1.3!.0!619.3-!(@*.-!/.2252'13.-!??A!
/.((.!*3)*31%(%!.-(!2#'*'0'8.!6.-!/.2252.-!*3)8%01(31/.-!
? 2'!/'*'/1(%!6.!*3)214%3'(1)0!>!.22.!.-(!6#'5('0(!*25-!1&*)3('0(.!=5.!2'!/.2252.!.-(!*25-!
6144%3.0/1%.:!
!
"'!3%852'(1)0!6.!2#$%&'()*)+,-.!.(!2#'6'*('(1)0!'5<!7.-)10-!4'1(!10(.39.013!2.-!facteurs#de#
croissance#hématopoïétiques:!B.!-)0(!6.-!/@()C10.-!6.!0'(53.!82@/)*3)(%1=5.:!"'!-*%/141/1(%!6#'/(1)0!
1&*21=5.!6.-!3%/.*(.53-!-*%/141=5.-!*3%-.0(-!-53!2.-!/.2252.-!/172.-:!
B.-!4'/(.53-!10(.391.00.0(!>!
? 6'0-!2.!&'10(1.0!6.!2#$)&%)-('-1.!
? 6'0-!2#'6'*('(1)0!D!/.3('10.-!-1(5'(1)0-!*'($)2)81=5.-!
!
Actuellement#seulement#le#G"CSF#et#l’EPO#sont#utilisés#en#thérapeutique.#
#
1. Le#G"CSF#naturel#=#rappels#:#
#
B@()C10.-!'@'0(!50.!'/(1)0!6.!facteur#de#croissance#hématopoïétique#restreint!-53!2.-!83'052.5<:!
E/(1)0!-53!2.-!*3%/53-.53-!0.5(3)*$12.-!
• Structure#:#
? 82@/)*3)(%10.!6.!FG!C;H!IJK!''!
? 2'!*'3(1.!82@/)-@2%.!3.*3%-.0(.!K!L!6.!2'!&)2%/52.!
#

!
!
-!
Cellules#productrices#:#
? 4173)72'-(.-!
? /.2252.-!.06)($%21'2.-!
? &)0)/@(.-!M!&'/3)*$'8.-!
? /.2252.-!-(3)&'2.-!6.!2'!&).22.!)--.5-.!
!
Cellules#cibles#:#
".!N?BOP!*)--,6.!50!3%/.*(.53!6.!$'5(.!'44101(%!-53!>!
? *3%/53-.53-!6.!2'!2180%.!83'052.5-.!0.5(3)*$12.!
? QR!0.5(3)*$12.-!&'(S3.-!
? B.3('10.-!/.2252.-!&'2180.-!
!
? Propriétés#physiologiques#:#
!
!
!
o !
o ".!N?BOP!-(1&52.!2'!proliférationH!la#différenciation#et#la#maturation!6.!2'!2180%.!
83'052)/@('13.!>!
61&105(1)0!65!6%2'1!6.!4)3&'(1)0!6#50!QR!&'(53.!>!IHT!U)53!'5!21.5!6.!J!U!
'58&.0('(1)0!65!0)&73.!6.!QR!*3)651(-!/$.V!2.!-5U.(!-'10!*'3!3'**)3(!'5!
-5U.(!0)0!(3'1(%!>!4'/(.53!IG!
o ".!N?BOP!&52(1*21.!par#100#le#nombre#de#cellules#souches#hématopoïétiques!
*3%-.0(.-!6'0-!2.!-'08!>!&)7121-'(1)0!*%31*$%31=5.!6.-!*3%/53-.53-!
!
!
o !
o ".!N?BOP!'58&.0(.!()5(.-!2.-!*)(.0(1'21(%-!65!QR!0.5(3)*$12.!>!
? *$'8)/@()-.!
? /@()()<1/1(%!E/?6%*.06'0(.!
? '/(191(%!7'/(%31/16.!6.-!QR!*'3!'58&.0('(1)0!6.!2'!*3)65/(1)0!6#1)0-!
-5*.3)<@6.-!
? &183'(1)0!6.-!QR!9.3-!2.-!(1--5-!*%31*$%31=5.-!D!*'3(13!65!-'08!
!
2. G"CSF#utilisés#en#thérapeutique#:#
#
2.1 G"CSF#recombinants#:#
#
2.1.1 Formes#commercialisées#:#
• Lenograstim#GRANOCYTE®#
? Q3)(%10.!6.!IJK!''!82@/)-@2%.H!F!*)0(-!61-5245.H!*3)651(.!.0!/.2252.-!6.!&'&&14,3.-!
? E6&101-(3'(1)0!WX!.(!OB!
? NYERZB[\]!I^!>!I^!_`W!a3%-.39%!D!2#5-'8.!*%61'(31=5.b!
? NYERZB[\]!^K!>!^K!_`W!
!
Action#sur#les#précurseurs#neutrophiles#
!
Action#sur#les#PN#neutrophiles#matures#
!

V"#39#FACTEURS#DE#CROISSANCE#HEMATOPOIETIQUES#–#CYTOKINES#ET#ANTAGONISTES#
!
!
"#$%&$'()*'+!
.!
• Filgrastim#NEUPOGEN®#
? Q3)(%10.!0)0!82@/)-@2%.!6.!IJT!''!*)--%6'0(!50.!&%($1)010.!-5**2%&.0('13.H!
*3)651(.!.0!/.2252.-!*3)/'3@)(.-!>!]:!/)21!
? E6&101-(3'(1)0!WX!.(!OB!
? R]`QZN]R!^G!>!^GG!c8!
? R]`QZN]R!Kd!>!KdG!c8!
!
Statut#administratif#:#
? 21-(.!W!
? ;1-*)0172.-!.0!)441/10.!
? Q3.-/31*(1)0!101(1'2.!$)-*1('21,3.!6.!^!&)1-!
!
2.1.2 Indications#thérapeutiques#:#
!
a)#Principe#général#:#
? "5((.3!/)0(3.!2.-!0.5(3)*%01.-!.0!'58&.0('0(!2.!0)&73.!.(!2'!4)0/(1)00'21(%!6.-!QR!0.5(3)*$12.-!
? Q.3&.((3.!50.!3%/5*%3'(1)0!*25-!3'*16.!6#50!('5<!'//.*('72.!6.!QR!
? Q3%9.013!2.!*310/1*'2!6'08.3!>!2'!0.5(3)*%01.!4%7312.!.(!-.-!/)0-%=5.0/.-!0%4'-(.-!a31-=5.!
104./(1.5<!&)3(.2H!'0(171)($%3'*1.!.(!$)-*1('21-'(1)0!
? E58&.0(.3!2.!/)04)3(!65!&'2'6.!.(!61&105.3!2.-!/)S(-!10651(-!*'3!2.-!%*1-)6.-!6.!0.5(3)*%01.!
!
b)#Indications#communes#aux#2#G"CSF#recombinants#:#
#
• Neutropénies#consécutives#à#une#chimiothérapie#cytotoxique#:#
o ".-!0.5(3)*%01.-!*.59.0(!e(3.!'//)&*'80%.-!6.!41,93.!'9./!&'U)3'(1)0!1&*)3('0(.!65!
31-=5.!104./(1.5<!
o ".-!%*1-)6.-!0.5(3)*%01=5.-!10651-.0(!50.!61&105(1)0!6.-!6)-.-!.(!50!.-*'/.&.0(!6.-!
/53.-!6.!/$1&1)!>!2#.441/'/1(%!65!(((!.-(!/)&*3)&1-.!
o ".!N?BOP!-.3'!*25-!*'3(1/521,3.&.0(!'--)/1%!D!2'!/$1&1)!6'0-!2.!/'-!6.!>!
\5&.53-!-)216.-!>!!
? B'0/.3!73)0/$1=5.!D!*.(1(.-!/.2252.-!
? B'0/.3!65!-.10!
? B'0/.3-!53)8%01('5<!
? B'0/.3!6.!2#)9'13.!
f%&'()2)81.!>!2@&*$)&.-!0)0!$)68C101.0-H!"E"!
o _)6'21(%-!6#'6&101-(3'(1)0!>!
R]`QZN]R!>!T!c8gC8gUH!9)1.!OB!)5!WX!
NYERZB[\]!>!ITG!c8g&FgU!)5!T!c8gC8gU!!H!9)1.!OB!
I!'6&1gU)53!
3.-*./(.3!50!10(.39'22.!&101&5&!6.!FK!$!'*3,-!2#'33e(!6.!2'!/$1&1)!a*)53!=5.!2.-!
0)59.'5<!QRR!*3)651(-!0.!-)1.0(!*'-!6%(351(-!*'3!2'!/$1&1)b!
;53%.!>!U5-=5#D!3%/5*%3'(1)0!6.-!QR!!-)1(!IG!D!IK!U!.0!3,82.!8%0%3'2.!

!
!
/!
!
!
"#'6&101-(3'(1)0!6.!N?BOP!.<)8,0.!'0052.!2'!-%/3%(1)0!6.!N?BOP!.06)8,0.!&'1-!*3)651(!50.!
3.&)0(%.!*25-!3'*16.!6.-!0.5(3)*$12.-!a^D!KUb:!B.!3'//)53/1--.&.0(!65!6%2'1!6.!3%/5*%3'(1)0!*.3&.(!
6.!21&1(.3!2.!6'08.3!6.!0.5(3)*%01.!4%7312.:!
!
!
!
o P6Y!1061=5'0(!50.!5(121-'(1)0!65!N?BOP!6,-!2'!I,3.!/53.!6.!/$1&1)!>!
? E\B;!6.!&'2'61.!0.5(3)*%01'0(.!
? ]*1-)6.!6.!0.5(3)*%01.!4%7312.!'5!/)53-!6#50!*3%/,6.0(!/@/2.!6.!/$1&1)!
? E\B;!6.!3'61)($%3'*1.!'5!019.'5!6.-!3%81)0-!31/$.-!.0!_Z!
? O5U.(!h8%H!E]N!
!
• Neutropénies#consécutives#à#la#thérapie#myélosuppressive#précédant#la#greffe#de#MO#:#
o R]`QZN]R!>!IG!c8gC8gU!U5-=5#D!6%*'--.&.0(!65!0'613!*51-!T!c8gC8gU!U5-=5#D!
0)3&'21-'(1)0!i!9)1.!OB!)5!WX!
o NYERZB[\]!>!ITG!c8g&FgU!i!9)1.!WX!
!
• Mobilisation#de#cellules#souches#progénitrices#dans#le#sang#circulant#(CSP)#:#
o I,3.!1061/'(1)0!>!&)7121-'(1)0!6.!/.2252.-!-)5/$.-!'5()2)85.-!.0!95.!6#'5()83.44.!>!
!"#$%&'&(&)&*+&,
-$./01&$23145$106&77
!"#$%&'()*%)#'+,(("-#%)./0"$+1+2/$,3&%(
8"$2&+&)&9&,:0%.
;<=">%?.&@%A&BC&DE%:2F

V"#39#FACTEURS#DE#CROISSANCE#HEMATOPOIETIQUES#–#CYTOKINES#ET#ANTAGONISTES#
!
!
"#$%&$'()*'+!
0!
N?BOP!-.52!)5!N?BOP!'*3,-!/$1&1)!2%8,3.!&@%2)-5**3.--19.!6#1065/()0!
I!10U:!OBgU)53!*6(!T!D!J!U)53-!/)0-%/5(14-!
O539.122'0/.!$%&'()!>!RPO!D!10(.39'22.-!3%8521.3-!
o F,&.!1061/'(1)0!>!&)7121-'(1)0!6.!BOQ!/$.V!2.!6)00.53!-'10!.0!95.!6#'22)83.44.!
N?BOP!5(121-%!-.52!>!IG!c8gC8gU)53!
Y1-=5.!'58&.0(%!6.!3%'/(1)0!65!83.44)0!/)0(3.!2#$j(.!*'3!3'**)3(!D!/.251!
10$%3.0(!D!2'!83.44.!6.!_Z!
#
c)#Indications#spécifiques#du#NEUPOGEN#:#
#
? Neutropénies#sévères#<#0,5.109/L#d’étiologies#variées#>!
B)08%01('2.-!>!6)-.!101(1'2.!6.!IF!c8gC8gU!*'3!9)1.!OB!.0!6)-.!501=5.!)5!43'/(1)00%.!
B@/21=5.!>!6)-.!101(1'2.!6.!T!c8gC8gU!*'3!9)1.!OB!.0!6)-.!501=5.!)5!43'/(1)00%.!
W61)*'($1=5.!>!6)-.!101(1'2.!6.!T!c8gC8gU!*'3!9)1.!OB!.0!6)-.!501=5.!)5!43'/(1)00%.!
E--)/1%.-!D!6.-!E\B;!6#104./(1)0-!-%9,3.-!
? Traitement#des#neutropénies#persistantes#<#109/L#chez#les#patients#infectés#par#le#VIH#à#un#
stade#avancé#
!
2.1.3 Effets#secondaires#:#
W2-!-)0(!'--.V!'(@*1=5.-!.(!61441/12.-!D!61-(1085.3!6.-!(3)572.-!21%-!'5<!*'($2)81.-!6'0-!2.!/'63.!
6.-=5.22.-!2.!N?BOP!.-(!*3.-/31(:!
? E//16.0(-!2.!*25-!43%=5.&&.0(!3.0/)0(3%-!>!
? ;)52.53-!)--.5-.-!aFGL!6.-!/'-b!
? P1,93.!
? Y%'/(1)0-!/5('0%.-!'5!*)10(!6#10U./(1)0!
? E//16.0(-!*25-!83'9.-!&'1-!3'3.-!>!
? E0'*$@2'<1.!
? k3)0/$)-*'-&.!
? O@063)&.!6.!451(.!/'*122'13.!
!
2.1.4 Contre"indications#:#
? N%0%3'2.-!>!
? E22.381.!
? f%&)*'($1.-!&@%2)+6.-!aBW!3.2'(19.b!
? W0(.0-141/'(1)0!/)0U)10(.!6.!2'!/$1&1)!
? E6&101-(3'(1)0!.0!même#temps!=5#50.!/$1&1)!/@()()<1=5.!
? O*%/141=5.-!>!
? Q)53!2.!R]`QZN]R!>!0.5(3)*%01.!/)08%01('2.!'9./!'0)&'21.!/@()8%0%(1=5.!
!
2.2 G"CSF#pégylé#:#
Pegfilgrastim#NEULASTA®##:#O)25(1)0!10U./('72.!OB!i!-.31085.!*3%?3.&*21.!i!l&8!
!
2.2.1 Structure#:#
? P)3&.!/)0U585%.!/)9'2.0(.!65!N?BOP!$5&'10!3./)&710'0(!41283'-(1&!'9./!50.!/$'10.!
210%'13.!6.!Q]N!a*)2@%($@2,0.?82@/)2b!-53!2#''!R?(.3&10'2!
? Q_!6.!^m!C;!
 6
6
 7
7
 8
8
 9
9
 10
10
 11
11
 12
12
 13
13
 14
14
 15
15
 16
16
 17
17
 18
18
 19
19
 20
20
 21
21
 22
22
 23
23
 24
24
 25
25
 26
26
 27
27
1
/
27
100%