loaded with in patients with advanced triple negative

Etude SAKK 24/14 befy 06.12.2016
Anti-EGFR-immunoliposomes loaded with doxorubicin in patients with
advanced triple negative EGFR positive breast cancer - A multicenter single arm
phase II trial
« Etude SAKK24/14 »
Sponsor : SAKK
CONTACTS AUX HUG :
Investigateur responsable: Dr Alexandre Bodmer, 079 55 32 405
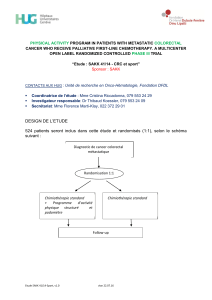
DESIGN DE L’ETUDE :
OBJECTIF DE L’ETUDE
Cette étude a pour but de déterminer l’efficacité de l’anti-EGFR-IL-dox en première ligne
chez des patients atteints d’un cancer du sein triple négatif et EGFR positif.
Un autre objectif est d’investiguer la sélection clonale sous ce traitement anti-EGFR-IL-dox
qui pourrait aboutir au développement d’une résistance au traitement. Pour ce faire des
prélèvements de sangs seront effectués à des temps précis au cours de l’étude.
CRITERES D’INCLUSION / EXCLUSION :
Main Inclusion criteria:
Metastatic or locally advanced non operable TNBC
EGFR expression of at least (1+) in immunohistochemistry
Measurable or evaluable disease
No prior systemic treatment for metastatic or inoperable disease
WHO PS of 0-2
Patient ≥ 18 years
Main Exclusion criteria:
Evidence of CNS or leptomeningeal metastases (even if previously treated)
History of hematologic or primary solid tumor malignancy, unless in
remission for at least 5 years. Inclusion of adequately treated cervical
carcinoma in situ or localized non-melanoma skin cancer is permitted
Previous therapy with more than 240 mg/m2 of doxorubicin or more than
450 mg/m2 of epirubicin
SAKK 24/14 - Anti-EGFR-IL-dox Trial summary, version 1.0, 10.05.2016 Page 2 of 6
Previous radiotherapy for the metastatic disease (palliative radiotherapy of
only non-target lesions is allowed)

Etude SAKK 24/14 befy 06.12.2016
Adjuvant treatment must have been stopped at least 6 months before
registration
Breastfeeding
Any other serious underlying medical, psychiatric, psychological, familial or geographical
condition, which in the judgment of the investigator may interfere with the planned staging,
treatment and follow-up, affect patient compliance or place the patient at high risk from
treatment-related complications.
1
/
2
100%