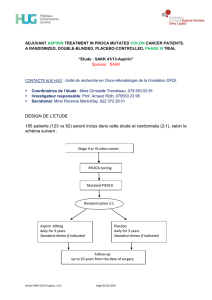
DESIGN DE L`ETUDE Environ 168 patients seront recrutés

Etude SAKK 08/14, V2.0, 20.06.16 dngn 21.12.2015
INVESTIGATION OF METFORMIN IN PATIENTS WITH CASTRATION RESISTANT
PROSTATE CANCER IN COMBINATION WITH ENZALUTAMIDE VS. ENZALUTAMIDE
ALONE, A RANDOMIZED, OPEN LABEL, PHASE II TRIAL
“Etude SAKK 08/14”
Sponsor : SAKK
CONTACTS AUX HUG : Unité de recherche en Onco-Hématologie, Fondation DFDL
Coordinatrice de l’étude : Mme Delphine Gani, 079 553 24 26
Investigateur responsable: Dr Anna Patrikidou, 079 553 60 99
Secrétariat: Mme Florence Marti-Klay, 022 372 29 01
DESIGN DE L’ETUDE
Environ 168 patients seront recrutés dans 15 sites et seront randomisés 1:1 dans l’un
des deux bras de traitement selon le schéma suivant :
mCRPC patients with:
- Progressive disease
under ADT
- Treatment Continued
androgendeprivation
OBJECTIF DE L’ETUDE
Evaluer l’efficacité de la combinaison Enzalutamide + Metformin, comparée à
l’Enzalutamide seul chez des patients ayant un cancer résistant à la castration et
progressant sous ADT.
Stratification factors:
- BMI
- Site of metastasis
- WHO performance status
Arm A :
Enzalutamide 160 mg
QD + Metformin 850
mg BID until PD
Arm B :
Enzalutamide 160 mg
QD until PD
Life long Follow-up

Etude SAKK 08/14, V2.0, 20.06.16 dngn 21.12.2015
CRITERES D’ELIGIBILITE
#
INCLUSION CRITERIA
YES
NO
1
Written informed consent according to ICH/GCP regulations before registration
and prior to any trial-related investigations
2
Histologically or cytological confirmed adenocarcinoma of the prostate without
small cell features
3
Asymptomatic or minimally symptomatic patients in relation to disease
4
Metastatic adenocarcinoma of the prostate documented by imaging (CT/MRI
and/or bone scan)
5
Ongoing androgen deprivation therapy with GnRH analogues or bilateral
orchiectomy (i.e. surgical or medical castration)
6
Total testosterone levels ≤ 1.7 nmol/L (corresponding to ≤ 50 ng/dL)
7
Tumor progression at the time of registration, defined as one or more of the
following three criteria that occurred while the patient was on androgen deprivation
therapy as defined in point 6.1.5:
PSA progression defined by a minimum of two rising PSA levels and an absolute
increase in total ≥ 2 ng/mL with an interval of ≥ 1 week between each determination.
Patients treated with an anti-androgen must have PSA progression after withdrawal
of previous treatment with e.g. flutamide, bicalutamide, nilutamide or
cyproteronacetate (≥ 6 weeks since last dose)
Soft tissue disease progression defined by RECIST 1.1
Bone disease progression defined by PCWG2 with two or more new lesions on bone
scan
8
Completed baseline QoL and pain questionnaires
9
Male patients ≥ 18 years
10
WHO performance status 0-2
11
Adequate hematologic values: hemoglobin ≥ 90 g/L, neutrophils ≥ 1.0 x 109/L,
platelets ≥ 75 x 109/L
12
Adequate hepatic function: ALT and AST ≤ 2.5 x ULN, bilirubin ≤ 1.5 x ULN
(exception if Gilbert’s syndrome ≤ 2.5 x ULN)
13
Adequate renal function: calculated creatinine clearance ≥ 50 mL/min, according
to the formula of Cockcroft-Gault
14
Patient is able to swallow the trial drugs and comply with trial requirements
15
Patient agrees not to father a child during participation in the trial and during 3
months thereafter
16
Patient agrees to participate in the 3 mandatory translational research projects on
blood samples, excepted the pyruvate dehydrogenase substudy

Etude SAKK 08/14, V2.0, 20.06.16 dngn 21.12.2015
#
EXCLUSION CRITERIA
YES
NO
1
Known or suspected CNS metastases or active leptomeningeal disease
2
Previous malignancy within 2 years prior to registration, with the exception of localized
non-melanoma skin cancer and Ta and Tis bladder cancer
3
Prior treatment for prostate cancer with
novel endocrine agents (including abiraterone acetate, enzalutamide, TAK-700, TAK-683,
TAK-448, VT464, ODM201, ARN509),
radioisotopes,
TKI and other small molecules,
immunotherapy or
chemotherapy (with the exception of docetaxel chemotherapy in hormone sensitive
prostate cancer)
4
Treatment with experimental drugs or treatment within a clinical trial within 30 days
prior to registration (except the clinical trial SAKK 96/12 and/or the biobank project
SAKK 63/12)
5
Clinically significant cardiovascular disease including:
Myocardial infarction within 6 months prior to registration,
Uncontrolled angina within 3 months prior to registration,
Congestive heart failure NYHA class III or IV
QTc interval > 480 ms,
History of clinically significant ventricular arrhythmias (e.g. ventricular tachycardia,
ventricular fibrillation, torsades de pointes),
History of Mobitz II second or third degree heart block without a permanent pacemaker in
place,
Uncontrolled hypertension as indicated by systolic blood pressure > 170 mmHg or
diastolic blood pressure > 105 mmHg
6
Severe concurrent disease, infection, or co-morbidity that, in the judgment of the
investigator, would make the patient inappropriate for enrollment (e.g. uncontrolled or
acute severe infection, advanced chronic obstructive pulmonary disease, heart failure)
7
Known history of HIV, hepatitis B, hepatitis C
8
Major surgery within 4 weeks prior to registration
9
Gastrointestinal disorder affecting absorption (e.g., gastrectomy, active peptic ulcer
disease within 3 months prior to registration)
10
Treatment with metformin within the last 6 months prior to registration
11
Patients on pharmacotherapy for diabetes mellitus
12
History of diabetic ketoacidosis, diabetic coma and pre-coma
13
Known history of seizures or any conditions that may predispose to seizure. History of
loss of consciousness or transient ischemic attack within 12 months prior to
registration
14
Concurrent anticoagulation with rivaroxaban or warfarin
15
Known hypersensitivity to the IMPs or hypersensitivity to any of their components
16
Any concomitant drugs contraindicated for use with the IMPs according to the
Swissmedic approved product information
17
Any psychological, familial, sociological or geographical condition potentially
hampering compliance with the trial protocol and follow-up
1
/
3
100%