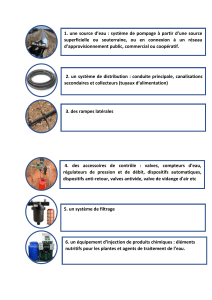
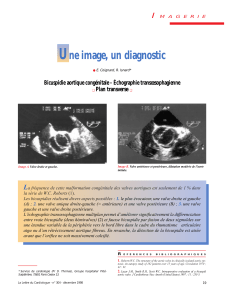
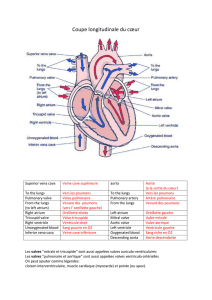
Sténose aortique & Cellules interstitielles : Impact du facteur tissulaire
Telechargé par
Laurent Jolia-Ferrier

UNIVERSITE LILLE 2 – DROIT ET SANTE
ECOLE DOCTORALE BIOLOGIE – SANTE
DOCTORAT DE BIOLOGIE CELLULAIRE,
BIOLOGIE DU DEVELOPPEMENT ANIMAL
Biologie Cellulaire
ANAIS ARBESU Y MIAR
CELLULE INTERSTITIELLE DE VALVE ET STENOSE AORTIQUE :
IMPACT DE LA VOIE DU FACTEUR TISSULAIRE.
Thèse dirigée par le Professeur Eric VAN BELLE
et le Docteur Delphine CORSEAUX.
Université Lille 2
Thèse soutenue le 16 décembre 2015
JURY
Professeur Thierry Le Tourneau Rapporteur
Professeur David Smadja Rapporteur
Docteur Delphine Corseaux Examinateur
Professeur Bart Staels Examinateur

A mes parents
qui n’ont jamais fait que s’assurer
de mettre toutes les chances de mon côté.
Merci à eux pour tous les sacrifices réalisés
en vue de m’assurer la concrétisation de mes projets.
Merci à eux d’avoir toujours cru en moi.
« On ne peut donner que
deux choses à ses enfants:
des racines et des ailes ».
-Proverbe juif-
A mes grands-parents.
« Un homme sans ancêtres
est un arbre sans racines,
un ruisseau sans source ».
-Proverbe chinois-
« L'impossible,
nous ne l'atteignons pas,
il nous sert de lanterne ».
René Char

Remerciements
J’aimerai tout d’abord remercier le Professeur Brigide Jude, le Professeur Sophie Susen
et le Professeur Bart Staels pour m’avoir accueilli au sein de leurs unités de recherche :
l’EA-2693 « interface sang vaisseaux et réparation cardiavosculaire » et l’UMR 1011
« Récepteurs nucléaire, Maladies Cardiovasculaires et Diabète ».
Je remercie également mes directeurs de thèse, le Professeur Eric Van Belle et le Docteur
Delphine Corseaux. Merci de m’avoir accueillie comme doctorante et de m’avoir fait
confiance tout au long de cette thèse. Merci également pour votre patience et votre soutien,
essentiels quand cela a été plus rude.
Je remercie bien évidemment mes rapporteurs de thèse, le Professeur Thierry Le
Tourneau de l’Université de Nantes et le Professeur David Smadja de l’Université Paris
Descartes. C’est un honneur de vous avoir dans mon jury de thèse.
Je remercie également l’Université Lille 2 et la Nouvelles Société Francophone
d’Athérosclérose (NSFA) pour m’avoir financée tout au long de cette thèse.
Je n’aurai jamais pu réaliser cette thèse si, à la fin de l’année 2011, le Docteur Joke Breyne
ne m’avait pas acceptée en stage au sein de l’EA-2693. Bien qu’en définitive, nous n’avons
pas travaillées ensemble ; sa confiance m’a permis d’intégrer cette équipe.
Je remercie également le Docteur Frédéric Mouquet. Merci pour les relectures et les
conseils qui ont permis l’amélioration indéniable de l’article « From human aortic valves to
valvular interstitial cells: in vitro behavior and cell subpopulation discrimination. » et de
cette thèse.
A mes collègues de l’EA-2693 et de l’UMR 1011, merci. Cette thèse est aussi le fruit d’un
travail collaboratif fructueux. A la VIC-team, merci à vous pour ces 3 années inoubliables.
Merci de m’avoir supportée lorsque j’en avais besoin, d’avoir partagé toutes les joies que
ces 3 années m’ont apportées et, surtout, d’avoir supporté mes coups de blues et mes
craintes grandissantes à l’approche de la fin. Ce fut jusqu’au bout un plaisir de venir au
laboratoire grâce à vous (même lors de la maroilles war).

Plus particulièrement, je remercie le Docteur Rodrigo Lorenzi. Au-delà de ton statut de
post-doctorant, l’aide que tu m’as apportée a permis la concrétisation de cette thèse.
Je remercie également Mickael Rosa pour m’avoir permis d’utiliser sa superbe figure
résumant la physiopathologie de la sténose aortique.
Plus personnellement, je remercie ma famille. Sans eux, je ne serais définitivement pas
arrivée là. Cela n’a pas toujours été facile mais, malgré la distance, vous avez toujours su
être là pour moi
A Simon, merci pour ton soutien sans faille tout au long de ces 2 années.
A mes colocataires : Marie, Charles, Mathieu ; merci pour l’année transition au sein de
cette coloc’ de grands malades. La soirée mexicaine restera ma préférée. A Katherini et
Maxime ; merci pour ses soirées tranquilles et nos raclettes-party. A Cécile et Charlotte ;
merci pour votre soutien dans la dernière ligne droite et dans l’adversité de ce week-end sans
chauffage. Merci à vous tous d’avoir rendu ma vie quotidienne lilloise si sympathique.
Et puis, « Il était une fois la VICs »…

RESUME EN ANGLAIS
Valvular interstitial cell and aortic stenosis: impact of tissue factor pathway
Defined as the narrowing of the aortic valve, aortic stenosis (AS) is the third cardiovascular
pathology in industrialized countries. Affecting mainly people aged over 65 years, AS
represents a major public health problem because of the aging of the population. After
initially been considered as a passive degenerative process, it is now established that AS is an
"atherosclerosis-like" disease characterized by the processes of inflammation, fibrosis,
neo-angiogenesis and calcification. Some proteins of the coagulation pathway such as tissue
factor (TF) are known to have a pro-fibrotic role and actively participate in the development
of atherosclerotic lesions. Their implication in AS seems, therefore, probable and remain to be
identified.
Prevalent cellular component of the aortic valve, VICs have five distinct subpopulations:
embryonic progenitor cells (EPCs), progenitor cells (pVICs) quiescent (qVICs), activated
(aVICs) and osteoblastic (obVICs). During the valvulogenesis, EPCs allow the cellularization
of the valve, differentiating into qVICs. These cells maintain the valvular homeostasis and, in
case of damage, are activated (aVICs) to effectively repair the valve tissue. The valvular
inflammation and VICs activation initiate the secretion of pro-calcifying proteins inducing the
differentiation of aVICs into obVICs. Finally, pVICs, naturally present within the valve
(called resident) or from the blood circulation (called hematopoietic), seem to promote cell
renewal and may be involved in the angiogenic and osteoblastic processes.
Although described, these subpopulations have never been studied longitudinally, in respect
to their behavior in vitro. Our first objective was to perform this investigation. Our second
objective was to study the potential role of TF pathway in the deleterious mechanisms of AS.
As part of the longitudinal follow-up of VICs from control and pathological human aortic
valves to the in vitro culture performed on plastic and collagen, we first showed that different
subpopulations were present in these valves with different locations and proportions
according to the pathophysiological state of the tissue. After enzymatic digestion, all
subpopulations are found but, in culture, hematopoietic pVICs disappeared, whichever the
support. Thus, we validated the primary culture model of VICs while highlighting its
limitations: lack of hematopoietic pVICs, spontaneous osteoblastic differentiation and
activation of VICs in culture.
 6
6
 7
7
 8
8
 9
9
 10
10
 11
11
 12
12
 13
13
 14
14
 15
15
 16
16
 17
17
 18
18
 19
19
 20
20
 21
21
 22
22
 23
23
 24
24
 25
25
 26
26
 27
27
 28
28
 29
29
 30
30
 31
31
 32
32
 33
33
 34
34
 35
35
 36
36
 37
37
 38
38
 39
39
 40
40
 41
41
 42
42
 43
43
 44
44
 45
45
 46
46
 47
47
 48
48
 49
49
 50
50
 51
51
 52
52
 53
53
 54
54
 55
55
 56
56
 57
57
 58
58
 59
59
 60
60
 61
61
 62
62
 63
63
 64
64
 65
65
 66
66
 67
67
 68
68
 69
69
 70
70
 71
71
 72
72
 73
73
 74
74
 75
75
 76
76
 77
77
 78
78
 79
79
 80
80
 81
81
 82
82
 83
83
 84
84
 85
85
 86
86
 87
87
 88
88
 89
89
 90
90
 91
91
 92
92
 93
93
 94
94
 95
95
 96
96
 97
97
 98
98
 99
99
 100
100
 101
101
 102
102
 103
103
 104
104
 105
105
 106
106
 107
107
 108
108
 109
109
 110
110
 111
111
 112
112
 113
113
 114
114
 115
115
 116
116
 117
117
 118
118
 119
119
 120
120
 121
121
 122
122
 123
123
 124
124
 125
125
 126
126
 127
127
 128
128
 129
129
 130
130
 131
131
 132
132
 133
133
 134
134
 135
135
 136
136
 137
137
 138
138
 139
139
 140
140
 141
141
 142
142
 143
143
 144
144
 145
145
 146
146
1
/
146
100%