Abstract book


Acknowledgements
Bio-Rad
Bioveta
Merial
Sanofi-Pasteur
Virbac
Joint OIE/WHO/EU International Conference: “Towards the Elimination of Rabies in Eurasia”
ii Paris (France), 27-30 May 2007

Contents
■ Introduction ---------------------------------------------------------------------------------- 1
■ Objectives of the Conference-------------------------------------------------------------- 2
■ Topics ----------------------------------------------------------------------------------------- 2
■ Organisation of the Conference ----------------------------------------------------------- 4
■ General Information------------------------------------------------------------------------- 4
■ Programme ----------------------------------------------------------------------------------- 5
■ Abstracts-------------------------------------------------------------------------------------- 11
KEYNOTE ADDRESS
Towards the elimination of rabies in Eurasia: overview and perspectives--------------------------- 12
J. Blancou
SESSION 1 – EPIDEMIOLOGICAL INFORMATION, RABIES IN EURASIA: REGIONAL REPORTS
Map: Areas selected for regional reports ---------------------------------------------------------------- 13
Rabies situation in Western Europe ----------------------------------------------------------------------- 14
A.I. Wandeler
Rabies situation in Eastern Europe------------------------------------------------------------------------- 15
O. Matouch
Rabies situation in Central Asia ---------------------------------------------------------------------------- 16
K.N. Gruzdev
Rabies situation in the Middle East ------------------------------------------------------------------------ 17
A. Seimenis
Rabies situation in Far East Asia -------------------------------------------------------------------------- 18
Z.F. Fu
SESSION 2 – RABIES PATHOGENESIS
Cytokine expression in the CNS and periphery during infection with rabies ------------------------ 19
N. Johnson, K.L. Mansfield, D. Hicks, A. Nunez, D. Healy, S.M. Brookes, C. McKimmie,
J.K. Fazakerley & A.R. Fooks
Neuroimaging, virus and cytokine studies in rabies infected dogs ----------------------------------- 20
J. Laothamatas, S. Wacharapluesadee, B. Lumlertdacha,S. Ampawong, V. Tepsumethanon,
P. Phumesin,S.Asavaphatiboon, L.Worapruekjaru, Y.Avihingsanon, N.Israsena & T.Hemachudha
Role of Calcitonin Gene-Related Peptide (CGRP) in the pathogenesis of rabies-------------------- 21
E. Weihe, M. Bette, M.A.R. Preuss, M. Faber, M.K. Schäfer, M.J. Schnell & B. Dietzschold
Pathogenic and attenuated rabies viruses induce differential host protein expression in
the central nervous system: implication of neuronal dysfunction ------------------------------------- 22
Z.F. Fu
Joint OIE/WHO/EU International Conference: “Towards the Elimination of Rabies in Eurasia”
Paris (France), 27-30 May 2007 iii

Dominance of a non-pathogenic over a pathogenic G protein gene in defining the
pathogenicity of a rabies virus------------------------------------------------------------------------------ 23
B. Dietzschold
SESSION 3 – RABIES PREVENTION AND CONTROL STRATEGIES
Can rabies be eradicated? (Keynote speech)------------------------------------------------------------- 24
C.E. Rupprecht, J. Barrett, D. Briggs, F. Cliquet, A.R. Fooks, B. Lumlertdacha, F.-X. Meslin,
T. Müller, L.Nel, C. Schneider, N. Tordo & A. Wandeler
Rabies Control in Dogs
Molecular epidemiology of lyssaviruses in Eurasia ----------------------------------------------------- 25
L.M. McElhinney, D. Marston, S. Stankov, C. Tu, C. Black, N. Johnson, Y. Jiang, N. Tordo,
T. Müller & A.R. Fooks
Identification of novel canine rabies virus clades in the Middle East and North Africa ----------- 26
D. David, G.J. Hughes, B.A. Yakobson, I. Davidson, H. Un, O. Aylan, I.V. Kuzmin & C.E. Rupprecht
Rabies in South Asia - Epidemiological investigation--------------------------------------------------- 27
C.K.Singh & B.S. Sandhu
Stray dogs in Bangkok, Thailand: rabies virus infection and rabies antibody prevalence -------- 28
S. Kasempimolporn, B. Sichanasai, W. Saengseesom, S. Puempumpanich,
S. Chatraporn & V. Sitprija
Preference for commercially produced oral rabies baits by feral dogs ------------------------------ 29
D. Bergman, S. Bender, D. Slate, C. Rupprecht, K. Wenning, C. Heuser, S. Joe & T. Deliberto
Assessment of the efficacy of oral vaccination of shepherd dogs in supplement of oral rabies
vaccination of wild canids in Israel------------------------------------------------------------------------ 30
B. A. Yakobson, R. King, N. Sheihat & D. David
Longterm immunization of recombinant rabies-canine adenovirus type-2 in dogs after
intranasal or oral vaccination------------------------------------------------------------------------------- 31
S.F. Zhang, Y. Liu, A.R. Fooks, F. Zhang & R.L. Hu
New steps in the control of canine rabies in India ------------------------------------------------------ 32
H.K. Pradhan, J.P. Gurbuxani, F. Cliquet, B. Pattnaik, S.S. Patil, A. Regnault, H. Begouen,
A.L. Guiot, R. Sood, P. Mahl, R. Singh, E. Picard, M.F.A. Aubert, J. Barrat & F.-X. Meslin
Rabies in Mexico, 1990-2005------------------------------------------------------------------------------ 33
C.H. Álvarez Lucas
Risk of rabies introduction by non-commercial movement of pets------------------------------------ 34
P. Have, l. Alban, L.T. Berndtsson, F. Cliquet, P. Hostnik, S.C. Rodeia & M. Sanaa
Rabies Control in Wildlife
Epidemiology of rabies in South East Europe------------------------------------------------------------- 35
N. Johnson, C. Freuling, A. Vos, H. Un, R. Valtchovski, M. Turcitu, F. Dumitrescu, V. Vlad,
R. Velic, V. Sandrac, O. Aylan, T. Müller & A.R. Fooks
Rabies in Mongolian steppes ------------------------------------------------------------------------------- 36
A.D. Botvinkin, D. Otgonbaatar, S. Tsoodol & I.V. Kuzmin
Epidemiology and control of rabies in Iran --------------------------------------------------------------- 37
A.R. Janani & A. Fayaz
Rabies epidemiology in raccoon dogs and foxes -------------------------------------------------------- 38
A. Singer, K. Kauhala, K. Holmala & G.C. Smith
Downside risk of wildlife translocation-------------------------------------------------------------------- 39
R. Chipman, D. Slate, C. Rupprecht & M. Mendoza
Sylvatic rabies in Russia with special attention to perspectives of oral vaccination of
wild carnivores ------------------------------------------------------------------------------------------------ 40
A. D. Botvinkin, D.V. Bankovsky & G.A. Safonov
Joint OIE/WHO/EU International Conference: “Towards the Elimination of Rabies in Eurasia”
iv Paris (France), 27-30 May 2007

Finnish-Russian collaboration programme on rabies control in wildlife: the outcome of
five years and the future prospects ------------------------------------------------------------------------ 41
A.E. Metlin, S.S. Rybakov, K.N. Gruzdev, V.V. Mikhalishin, A. Huovilainen & E. Neuvonen
Rabies eradication programme in Estonia by means of oral vaccination of wildlife: first
results----------------------------------------------------------------------------------------------------------- 42
E. Niin, M. Laine & A. Pärtel
Rabies surveillance in Poland between 1992 - 2006--------------------------------------------------- 43
M. Smreczak, P. Trębas, A. Orlowska & J.F. Żmudziński
Efficacy of a square V-RG vaccine baits in red fox, domestic dog and raccoon dog--------------- 44
F. Cliquet, A.L. Guiot, C. Schumacher, J. Maki & J. Barrat
Scenario-analysis evaluating emergency strategies after rabies re-introduction ------------------ 45
H.-H. Thulke, D. Eisinger, T. Selhorst & T. Müller
The financial challenge to keep a large region free of rabies – the EU example------------------- 46
C. Freuling, T. Selhorst & T. Müller
What is the future of rabies control in Europe? ---------------------------------------------------------- 47
G.C. Smith, H.-H. Thulke, A.R. Fooks, M. Artois, D.W. Macdonald, D. Eisinger & T. Selhorst
The strength of 70% - revising a given threshold of rabies control ---------------------------------- 48
H.-H. Thulke & D. Eisinger
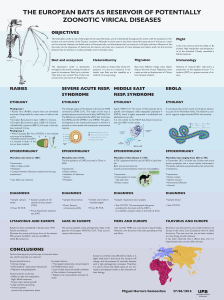
Rabies in Bats: an emerging zoonosis
A random grid based molecular epidemiological study on EBLV isolates from Germany---------- 49
C. Freuling, T. Selhorst, L. Geue, D. Marston, N. Johnson, A.R. Fooks, N. Tordo & T. Müller
Asymptomatic rhabdovirus infection in meridional serotine bats (
Ep esicus isabel nus
)
t li
from Spain------------------------------------------------------------------------------------------------------ 50
J.E. Echevarría, S. Vázquez-Morón, C. Ibáñez, J.C. Aznar, E. Ruiz & J. Juste
Public health hazard of European bat lyssavirus, the Netherlands ----------------------------------- 51
W.H.M. Van Der Poel, K. Takumi, E.R.A.M. Verstraten, P.H.C. Lina,
J. Van Der Giessen & J.A. Kramps
Serological investigation of bats infected with rabies virus in China -------------------------------- 52
L. Wang, Y. Jiang, Z. Lu, K. Sun, H. Xuan, A. Fooks & C. Tu
Epidemiology and pathogenicity of African bat lyssaviruses------------------------------------------- 53
W. Markotter, C. Wilsenach & L.H. Nel et al.
Experimental infection of big brown bats (
Eptesicus fuscus
) with West Caucasian
bat virus -------------------------------------------------------------------------------------------------------- 54
I.V. Kuzmin, R. Franka & C.E. Rupprecht
Susceptibility of foxes (
Vulpes vulpes
) to European bat lyssaviruses types-1 and -2 ------------- 55
F. Cliquet, E. Picard, J. Barrat, S.M. Brookes, D.M. Healy & A.R. Fooks
Identification of British bat species using sequence comparison and its application to
European bat lyssavirus diagnosis and surveillance ---------------------------------------------------- 56
S.L. Harris, N. Johnson, S.M. Brookes, A.R. Fooks & G. Jones
Rabies Prevention in Humans
Human rabies includes early generalized vasospasm of cranial arteries that responds to
tetrahydrobiopterin, l-arginine or nitroprusside---------------------------------------------------------- 57
R.E. Willoughby, A. Roy-Burman, K.W. Martin, J.C. Christensen, D.F. Westenkirchner, C. Glaser,
C.E. Rupprecht & K. Hyland
Therapy of human rabies: lessons from experimental studies in a mouse model------------------- 58
A.C. Jackson, C.A. Scott, J. Owen, S.C. Weli & J.P. Rossiter
Rabies control and prevention in Georgia: current status and perspectives------------------------- 59
P. Imnadze, V. Surguladze, T. Tushishvili & l. Baidoshvili
Joint OIE/WHO/EU International Conference: “Towards the Elimination of Rabies in Eurasia”
Paris (France), 27-30 May 2007 v
 6
6
 7
7
 8
8
 9
9
 10
10
 11
11
 12
12
 13
13
 14
14
 15
15
 16
16
 17
17
 18
18
 19
19
 20
20
 21
21
 22
22
 23
23
 24
24
 25
25
 26
26
 27
27
 28
28
 29
29
 30
30
 31
31
 32
32
 33
33
 34
34
 35
35
 36
36
 37
37
 38
38
 39
39
 40
40
 41
41
 42
42
 43
43
 44
44
 45
45
 46
46
 47
47
 48
48
 49
49
 50
50
 51
51
 52
52
 53
53
 54
54
 55
55
 56
56
 57
57
 58
58
 59
59
 60
60
 61
61
 62
62
 63
63
 64
64
 65
65
 66
66
 67
67
 68
68
 69
69
 70
70
 71
71
 72
72
 73
73
 74
74
 75
75
 76
76
 77
77
 78
78
 79
79
 80
80
 81
81
 82
82
 83
83
 84
84
 85
85
 86
86
 87
87
 88
88
 89
89
 90
90
 91
91
 92
92
 93
93
 94
94
 95
95
 96
96
 97
97
 98
98
 99
99
 100
100
 101
101
 102
102
 103
103
 104
104
 105
105
 106
106
 107
107
 108
108
 109
109
 110
110
 111
111
 112
112
 113
113
 114
114
 115
115
 116
116
 117
117
 118
118
 119
119
 120
120
 121
121
 122
122
 123
123
 124
124
1
/
124
100%