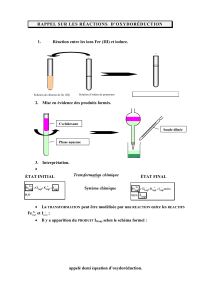
Recycling of CO2: an alternative to

Congrès de la Société Chimique de France – 2015
SCF Congress - 2015
Recycling of CO2: an alternative to petrochemistry for the
synthesis of nitrogen compounds
Recyclage du CO2 : une alternative à la pétrochimie pour la synthèse
de composés azotés
E. Blondiaux1,2, X. Frogneux1, J. Pouessel1, T. Cantat*,1
1 CEA, 91191 Gif-sur-Yvette
2 ADEME, 49004 Angers
* Corresponding author: Thibault[email protected]
______________________________________________________________
Résumé : L'utilisation du CO2 pour la production de molécules contenant des atomes C1 est une voie attrayante
pour la synthèse de produits chimiques à valeur ajoutée. En particulier, la formation de composés azotés peut être
obtenue par fonctionnalisation réductrice du CO2 en présence d'amines. En utilisant des réducteurs doux tels que
des hydrosilanes et des hydroboranes, de nouvelles réactions ont été conçues pour faciliter la transformation du CO2
en dérivés formamides, formamidines, aminals et méthylamines, par voies organocatalytiques.
________________________________________________________________________
Summary: CO2 utilization for the production of C1-containing molecules is a desirable route to value-added
chemicals. In particular, formation of nitrogen compounds can be obtained by reductive functionalization of CO2 in the
presence of amines. Using mild reductants, such as hydrosilanes and hydroboranes, novel catalytic reactions have
been designed to facilitate the transformation of CO2 to formamide, formamidine, aminal and methylamine
derivatives, by organocatalysis.
Keywords: CO2; reduction; catalysis; sustainable chemistry; nitrogen compounds
While the CO2 concentration in the atmosphere continues to grow, the search for technologies to reduce CO2 emissions
is urgent. Among the solutions explored, CO2 transformation into fuels and chemicals has emerged as an alternative to
CO2 capture and storage for reducing the emissions of this greenhouse gas. CO2 conversion to chemicals might offer
niche applications in the short term, because the endproducts possess an added-value able to balance the cost of CO2
capture and transformation.
Alors que la concentration de CO2 dans l'atmosphère continue d’augmenter, la recherche de technologies pour réduire
les émissions de CO2 est urgente. Parmi les solutions explorées, la transformation du CO2 en carburants et produits
chimiques a émergé comme une alternative à la capture et au stockage du CO2 pour réduire les émissions de ce gaz à
effet de serre. La conversion du CO2 en produits chimiques pourrait offrir des applications de niche à court terme, car les
produits finaux possèdent une valeur ajoutée en mesure d'équilibrer le coût du captage du CO2 et de sa transformation.
1 Introduction
CO2 conversion into chemicals is an attractive
opportunity for CO2 recycling and has therefore
received a wide attention over the last few years.[1]
Using amines as functionalizing reagents, synthesis
of nitrogen compounds have been developed.
These novel processes are able to transform CO2
into formamide, formamidine, aminal and
methylamine derivatives, which are important
moieties in synthetic chemistry (fig. 1.). For
example, formamidines derivatives are useful
chemicals because they are utilized as antifungal,
antibacterial, anticancer and anticonvulsant agents;
methylamines are valuable for the synthesis of a
broad range of products applicable in such diverse
fields as medicine, agriculture, rubber, plastics and
synthetic fibers. In particular, monomethylamine,
dimethylamine and trimethylamine are produced
each year at about 500 000 t.
Fig. 1. Nitrogen compounds available from CO2 and amines
under organocatalysis and hydrosilylation or hydroboration
conditions
2 Experimental/methodology
The thermodynamic stability of CO2 imposes an
input of energy to convert CO2 into chemicals. The
second challenge is kinetic in nature: catalysts are
required to ensure that the activation barriers
remain as low as possible along the chemical

transformation pathways so that the overall carbon
balance for CO2 utilization is not hampered by
thermal loading needed to overcome high energy
transition states.
Obviously, these energetic considerations are
associated with strong constraints on the resources
utilized for CO2 conversion as the energy input
must be carbon-free and rare or toxic metal
catalysts must be avoided.
In this regard, in order to convert CO2 into
chemicals, mild reductants have been employed
such as hydrosilanes (Si–H bonds) and
hydroboranes (B–H bonds). Among them, PMHS
(polymethylhydrosiloxane) is particularly attractive
because it is a cost-efficient, non-toxic and air
stable waste of the silicon industry.
Furthermore, organocatalysts like nitrogen or
phosphorus bases have been considered because
they usually combine low cost and low toxicity with
an enhanced stability to moisture and air, which can
circumvent classical drawbacks of many metallic
catalysts.
Fig. 1. Organocatalysts and reductants used in this contribution:
Verkade’s base (VB), TBD (1.5.7-triazabicyclo[4.4.0]des-5-ene),
PMHS (polymethylhydrosiloxane), 9-BBN (9-borabicyclo[3.3.1]
nonane)
3 Results and discussion
Under organocatalysis conditions, five
processes for CO2 conversion are highlighted.
Using a Verkade base and PMHS, a 2–electron
reduction to formamides and formamidines has
been achieved.[2] Aminals have been also
synthesized with short reaction time (< 6 h).[3]
Finally, the 6–electron reduction of CO2 to
methylamines, a process unveiled in 2013, was
developed for the first time under metal-free
conditions.[4] Interestingly, the reaction under
hydroboration conditions exhibits better activities
than the precedent reported processes using Zn or
Ru based catalysts for this reaction.[5]
Table 1 Processes for CO2 conversion into nitrogen compounds
Nitrogen
compound
Catalyst
Reductant
Conditions
Formamides
VB
PMHS
24 h / 20 °C
Formamidines
VB
PMHS
24 h / 70 °C
Aminals
TBD
PhSiH3
6 h / 80 °C
Methylamines
VB
9-BBN
1 h / 90 °C
Methylamines
VB/B(C6F6)3
PMHS
24 h / 100 °C
4 Conclusions
In conclusion, we have developed
unprecedented methods for the creation of nitrogen
compounds with CO2. These transformations
enable the functionalization of a large scope of
substrates, including aliphatic and aromatic amines,
with a high chemoselectivity.
Acknowledgements
For financial support of this work, we
acknowledge the CEA, CNRS, ADEME, the
CHARMMMAT Laboratory of Excellence and the
European Research Council (ERC Starting Grant
Agreement n.336467). T.C. thanks the Foundation
Louis D. – Institut de France for its formidable
support.
References
[1] (a) A. Goeppert, M. Czaun, J.-P. Jones, G. K. Surya
Prakash, G. A. Olah, Chem. Soc. Rev. 43 (2014) 7995. (b)
F. J. Fernandez-Alvarez, A. M. Aitani, L. A. Oro, Catal. Sci.
Technol. 4 (2014) 611. (c) A. Tlili, E. Blondiaux, X.
Frogneux, T. Cantat, Green Chem. 17 (2015) 157. (d) A.
Tlili, X. Frogneux, E. Blondiaux, T. Cantat, Angew. Chem.
Int. Ed. 53 (2014) 2543.
[2] E. Blondiaux, T. Cantat, submitted, (2015).
[3] X. Frogneux, E. Blondiaux, P. Thuery, T. Cantat, submitted,
(2015).
[4] E. Blondiaux, J. Pouessel, T. Cantat, Angew. Chem. Int. Ed.
53 (2014) 12186.
[5] (a) O. Jacquet, X. Frogneux, C. Das Neves Gomes, T.
Cantat, Chem. Sci. 4 (2013) 2127. (b) Y. Li, I. Sorribes, T.
Yan, K. Junge, M. Beller, Angew. Chem. Int. Ed. 52 (2013)
12156. (c) K. Beydoun, T. vom Stein, J. Klankermayer, W.
Leitner, Angew. Chem. Int. Ed. 52 (2013) 9554.

Congrès de la Société Chimique de France – 2015
SCF Congress - 2015
New Glycerol-derived Hydrotropes for Low Temperature
Cloud Point Extraction Processes.
Nouveaux Hydrotropes à base de Glycérol pour des Procédés
d’Extractions à Points Troubles à Basse Température.
R. Lebeuf1, E. Illous2, V. Nardello-Rataj2, J.-M. Aubry1*
1 ENSCL, Cité Scientifique, Avenue Mendeleïev, 59652 Villeneuve D’Ascq
2 Université de Lille, UCCS, équipe CISCO, bât C6, F-59655 Villeneuve d’Ascq CEDEX
* Corresponding author: jean-marie.aubry@ensc-lille.fr
______________________________________________________________
Résumé : des dialkyl-éthers de glycerols avec de courtes chaînes carbonées ont été synthétisés afin d’étudier leurs
propriétés hydrotropiques. En plus de leur bon pouvoir solubilisant envers les molécules hydrophobes, ils présentent
en solutions aqueuses des points de troubles à basses températures sur une large gamme de concentrations, ce qui
les rend potentiellement intéressants pour des procédés d’extractions moins énergivores. Ils peuvent ainsi être de
bons substituts aux hydrotropes pétrosourcés tels que le C4E1 et les sulfonates d’alkylbenzenes, comme démontré
avec l’extraction de la pipérine du poivre.
_______________________________________________________________________
Summary: Some glycerol dialkylethers with short alkyl chains have been synthesized to study their hydrotropic
properties. In addition to their good properties for the solubilization of hydrophobic molecules, they possess in
aqueous solutions low temperature cloud points on a large range of concentrations, making them potentially suitable
for lower energy demanding extraction processes. Hence, they could be good alternatives to petro-based
hydrotropes like C4E1 or alkyl-benzensulfonates, as demonstrated by the extraction of the piperine from pepper.
Keywords: hydrotrope, glycerol, extraction, cloud point, piperine.
Les produits naturels sont de plus en plus plébiscités, cependant leur extraction du milieu naturel peut être
problématique pour cause de dégradations lors de chauffages ou de purifications supplémentaires couteuses et
énergivores. Les procédés d’extractions à base d’hydrotropes sont des alternatives modernes plus respectueuses
envers ces critères. Néanmoins, pour cela, de nouvelles molécules si possibles agro-sourcées restent à être identifiées,
ce qui fait l’objet de ce travail illustré par l’extraction de la pipérine présente naturellement dans le poivre.
1 Introduction
Hydrotropes are small amphiphilic compounds
composed of a polar head and a short hydrophobic
tail that distinguish them from surfactants which
possess longer tails. They could be used for many
applications such solubilization of high value
hydrophobic compounds, for the formation of
microemulsions in presence of surfactants, and for
extraction processes like shown in a recent study
showing the separation of lignine from bagasse of
sugar cane, that may allow to get purer lignic and
cellulosic materials for bioenergetic conversions by
this way.[1]
Extraction processes are generally performed
using solvents, which have to be separated by
distillation. Selectivity of the extraction is also a
critical parameter to reduce waste and costly
purifications. In response to these problems,
hydrotropes allows using water as solvent and
could enhance extraction selectivity. The solubilized
product could be then separated by simple dilution,
however, the use of a large amount of water
impede the easy recovery of the hydrotrope. Cloud
Point Extraction (CPE) process could avoid such
problems. Indeed, when a homogeneous aqueous
solution of a hydrotrope having a cloud point is
heated above this temperature, the hydrotrope is
released from water. If a solute would also be
present, it will go in the organic layer formed by the
hydrotrope, which can be then more easily recover.
This concept was first applied for the extraction of
metals, but could be extended for organic
compounds.
2 Synthesis and properties of a
dialkylglycerol ether
A glycerol dialkylether could be easily obtained
from butyl glycidyl ether, a largely available product
due to its use in epoxy resins. Simple acid-
catalyzed ring opening of the epoxide in methanol
give a mixture of the two regioisomers in 4:1 ratio.
Even if the regioisomers could be separated, the

present study was done on the mixture to proof its
scalability.
The resulting mixture of alcohols is miscible in
water in all proportions at room temperature. As
shown in figure 1, it present cloud points in water, a
rare phenomenon for non-ionic compounds other
than polyoxoethylenes. [2]
Fig. 1. Cloud points of a dialkylglycerol mixture compared to
C4E1 in function of the weight fraction of the hydrotrope.
The solubilizing properties of a hydrotrope for
hydrophobic compounds are characterized by a
rapid increase of the amount of solubilized product
from a certain concentration of the hydrotrope
called Minimum Hydrotropic Concentration (MHC).
That is observed in our case using Disperse Red13,
dye and the solubilization was more pronounced
compared to the corresponding mono alkylglycerol
(Figure 2).
Fig. 2. Solubilization of hydrophic disperse red 13 at room
temperature by two hydrotropes in water. Comparison of mono
and dialkyl glycerol ether.
3 Extraction of piperine from pepper
A solution (2M) of the synthesized hydrotropes
was stirred with black pepper to see whether they
could be a good alternative for alkylbenzene
sulfonates used for such application. [3] The
extraction was monitored by HPLC at 340 nm (max
piperine) and 254 nm. A clean extraction is
observed, allowing quantifying the amount of
extracted piperine (Figure 3).
Fig. 3. Extraction chromatogram (after 4 hours) and amount of
extracted piperine from black pepper at room temperature with a
2M solution of hydrotropes in water (mixture of isomers of butyl-
methyl glycerol diether)
Like for alkylbenzene sulfonates, [3] extraction
is not linear along the time. Presence of hydrotrope
increase the amount of solubilized piperine
compared to a blank experiment done with only
water. Hence, piperine could be extracted and
easily recovered along the hydrotrope by heating
the solution above the cloud point.
4 Conclusions
Dialkyl glycerol ethers are good candidates for
Cloud Point Extraction processes (CPE) and
variation of the substituents could tune the thermal
and solubility properties of such compounds for
more specific applications.
Acknowledgements
The authors thank the Université de Lille and the
Ecole Nationale Supérieure de Chimie de Lille for
financial supports.
References
[1] K. B. Ansari, V. G. Gaikar Chem. Eng. Sci. 115 (2014) 157.
[2] A. Lavergne, L. moity, V. Molinier, J.-M. Aubry RSC
Advances 3 (2013) 5997.
[3] G. Raman, V. G. Gaikar Ind. Eng. Chem. Res. 41 (2002)
2966.
0
20
40
60
80
100
020 40 60 80 100
Temperature ( C)
Hydrotrope in water (%wt)
(C4E1)
miscibility domain
non miscibility
domain
0
2
4
6
8
10
12
0 0,5 1 1,5 2
solubilized Disperse Red 13
(mmol.L-1)
Hydrotrope (mol.L-1)
MHC
-50000
0
50000
100000
150000
200000
250000
300000
010 20 30 40 50 60 70 80
relative intensity
Time / Min.
340 nm
254 nm
piperine
0
2
4
6
8
10
12
0 2 4 6 8 10
Piperine in solution (mg/g)
Extraction time (h)
without hydrotrope

Congrès de la Société Chimique de France – 2015
SCF Congress - 2015
Study of prompt NO formation during methyl esters
combustion in low pressure premixed flames
Etude de la formation du NO précoce lors de la combustion des esters
méthyliques à basse préssion et en conditions de flammes plates
prémélangées
M.D. Sylla*, L. Gasnot, N. Lamoureux
Université de Lille, Laboratoire de PhysicoChimie des Processus de Combustion et de
l’Atmosphère PC2A UMR 8522, Bâtiment C11, 59655 Villeneuve d’Ascq Cedex France
* Corresponding author: marame-diamb.sylla@ed.univ-lille1.fr
______________________________________________________________
Résumé : Ce travail porte sur l'étude de l'impact environnemental des esters méthyliques utilisés comme biodiesel et
concerne plus particulièrement la cinétique de formation des oxydes d'azote (NOx) dans des conditions de flamme. Il
s’agit de tester et d'optimiser un mécanisme cinétique détaillé dédié à la prédiction de la formation des NOx lors de
l'oxydation du méthyle de butanoate, composé modèle représentatif du biodiesel. Pour cela, les profils d’un large
panel d’espèces chimiques ont été mesurés dans un brûleur de laboratoire, et comparés à ceux obtenus par les
mécanismes cinétiques détaillés disponibles dans la littérature. Les mesures sont réalisées par couplage des
techniques de spectroscopie laser et de techniques d'analyse classiques.
________________________________________________________________________
Summary: This work is focused on the study of the environmental impact of methyl esters used as biodiesel and
concerns more particularly the kinetic of nitrogen oxides formation in flame conditions. The aim of this study is to test
and optimize detailed kinetic mechanism for the prediction of NOx formation during the oxidation of methyl butanoate,
a compound chosen as model for biodiesel. For that, the profiles for a wide range of chemical species were
measured in a laboratory burner, and compared with those obtained by detailed kinetic mechanisms available in the
literature. Measurements are performed by coupling laser diagnostic techniques and classical analysis techniques.
Keywords: Prompt-NO; Methyl esters; Combustion; Premixed flame; kinetic chemistry;
Environmental concerns lead to the development of biofuels as renewable energy. Methyl esters issued from chemical
transformation from biomass are used as additives to fossile energy (diesel or gasoline). These additives allow limiting
greenhouse emissions. However, very few data are available concerning their oxidation impact on NOx emissions.
Les préoccupations liées à la pollution atmosphérique ont conduit à l’accroissement de l’utilisation d’agrocarburants. Les
esters méthyliques, issus de la transformation chimique de la biomasse, peuvent être utilisés comme additifs aux
carburants fossiles (diesel ou essence). Ces additifs présentent l’avantage de limiter les émissions de gaz à effet de
serre. Cependant, peu de données ont été réalisées pour caractériser l’impact de leur oxydation sur le plan des
émissions de NOx.
1 Introduction
Due to the increase of demand for energy,
concerns for the oxidation of fuels impact on
environment and healthy have been reviewed since
the late 90’s. Concerning the reduction of pollutant
emission due to transport, one option relies on the
use of biodiesels that are typically derived from oil
transesterification.
Because of the complexity of biodiesel, methyl
butanoate (MB) which is the simplest methyl ester
is a very good candidate to examine the chemical
kinetics aspect of the ester oxidation.
In the present work, NO formation was studied
during MB oxidation in premixed low pressure
flames. Experimental conditions were selected to
examine the prompt-NO formation exclusively. NO
species and temperature profiles were measured in
situ by using LIF (Laser-Induced Fluorescence)
techniques. Stable species profiles were measured
after gas probe sampling by using analytical
techniques such as GC (Gas Chromatography) and
FTIR (Fourier Tranform InfraRed) spectroscopy.
Experimental results have been compared to
species profiles calculated by using Premix code
with appropriated detailed kinetics mechanisms.
For that purpose, detailed mechanisms available in
the literature [1, 2] were updated with the NOx
chemistry extracted from Lamoureux et al. [3].
2 Experimental/methodology
Structure analysis in laminar low pressure
flames consists in measuring absolute species
profiles along the vertical axis above the burner.
Due to the flame configuration, the vertical axis is
representative of the chemical time scale
connected to the time scale of the reactions.
Five MB/CH4/O2/N2 premixed flames were
stabilized on a McKenna burner in low pressure
 6
6
 7
7
 8
8
 9
9
 10
10
 11
11
 12
12
 13
13
 14
14
 15
15
 16
16
 17
17
 18
18
 19
19
 20
20
 21
21
 22
22
 23
23
 24
24
 25
25
 26
26
 27
27
 28
28
 29
29
 30
30
 31
31
 32
32
 33
33
 34
34
 35
35
 36
36
 37
37
 38
38
 39
39
1
/
39
100%


![Suggested translation[1] He learned[2] to dress tastefully. He moved](http://s1.studylibfr.com/store/data/005385129_1-269daba301ff059de68303e1bc025887-300x300.png)