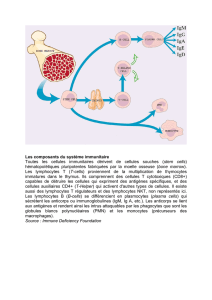
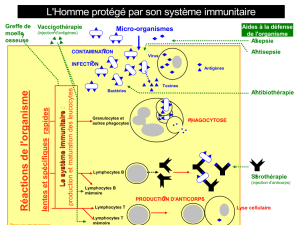
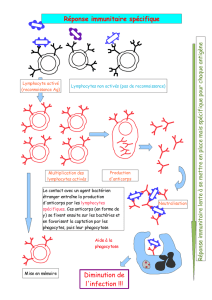
Guide des anticorps monoclonaux et protéines de fusion à usage

Guide des anticorps monoclonaux et protéines de fusion
à usage thérapeutique
Hélène KAPLON
Docteur en Pharmacie
Christophe CARNOY
Maître de conférences
Service d’Immunologie
Faculté des Sciences Pharmaceutiques et Biologiques de Lille
Version Mars 2015

1

2
AVANT PROPOS
Ce guide a pour but de faire une synthèse des anticorps monoclonaux (AcM) et dérivés à
usage thérapeutique ayant obtenu une AMM en France ou en Europe. Après quelques généralités
sur les anticorps et le mode d’obtention de ces molécules, les AcM thérapeutiques seront présentés
en fonction de leur cible ou de leurs indications. Enfin, les caractéristiques de chaque AcM
thérapeutique seront présentées sous forme de fiches résumées où seront notamment détaillés les
posologies et les mécanismes d’action. Compte tenu de l’évolution rapide du domaine des
biothérapies en général et des anticorps monoclonaux en particulier, une mise à jour semestrielle
sera, dans la mesure du possible, effectuée. Les questions et les commentaires sont les bienvenus et
sont à transmettre à [email protected].
Ont contribué à ce document :
• Hélène Kaplon (helene.kaplon@gmail.com), docteur en Pharmacie pour la veille scientifique
• Dr Christophe Carnoy (christop[email protected]r), Maître de Conférences en
immunologie, pour la conception et l'organisation du guide
• Drs Nadine Roger, Emmanuel Hermann et Benjamin Bertin, Maîtres de Conférences dans le
service d'immunologie de la Faculté des Sciences Pharmaceutiques et Biologiques de Lille,
pour leur expertise
Les sources d’information utilisées dans le document :
• Le site de l’IMGT (http://www.imgt.org/mAb-DB/index) correspond à la meilleure source
d’information disponible à ce jour sur les anticorps monoclonaux.
• Le Vidal (http://www.evidal.fr)
• Les sites de l’HAS (http://www.has-sante.fr), l’EMA (http://www.ema.europa.eu) ainsi que
Pubmed
Aucune copie de ce document n’est autorisée sans l’autorisation préalable du service
d’immunologie de la faculté des Sciences Pharmaceutiques et Biologiques de Lille.

3
TABLE DES MATIERES
AVANT PROPOS ...........................................................................................................................2
TABLE DES MATIERES ..................................................................................................................3
LISTE DES ABREVIATIONS ............................................................................................................5
GENERALITES ..............................................................................................................................8
LISTE PAR PATHOLOGIE ............................................................................................................. 12
LISTE PAR ISOTYPE .................................................................................................................... 22
LISTE PAR CIBLE THERAPEUTIQUE ............................................................................................. 23
CARACTERISTIQUES DES ANTICORPS MONOCLONAUX THERAPEUTIQUES ................................. 31
Abciximab (REOPRO®) ........................................................................................................ 32
Adalimumab (HUMIRA®) .................................................................................................... 33
Alemtuzumab (LEMTRADA®) .............................................................................................. 34
Basiliximab (SIMULECT®) .................................................................................................... 35
Belimumab (BENLYSTA®) ................................................................................................... 36
Bevacizumab (AVASTIN®) .................................................................................................. 37
Brentuximab vedotin (ADCETRIS®) ..................................................................................... 38
Canakinumab (ILARIS®) ..................................................................................................... 39
Catumaxomab (REMOVAB®) .............................................................................................. 40
Certolizumab pegol (CIMZIA®) ............................................................................................ 41
Denosumab (PROLIA®, XGEVA®) ......................................................................................... 42
Eculizumab (SOLIRIS®) ........................................................................................................ 43
Golimumab (SIMPONI®) ..................................................................................................... 44
Ibritumomab (ZEVALIN®) .................................................................................................... 45
Infliximab (REMICADE®) ..................................................................................................... 46
Ipilimumab (YERVOY®) ....................................................................................................... 47
Natalizumab (TYSABRI®) ..................................................................................................... 48
Obinutuzumab (GAZYVARO®) ............................................................................................. 49
Ofatumumab (ARZERRA®) .................................................................................................. 50
Omalizumab (XOLAIR®) ...................................................................................................... 51
Palivizumab (SYNAGIS®) .................................................................................................... 52
Panitumumab (VECTIBIX®) ................................................................................................. 53
Pertuzumab (PERJETA®) ..................................................................................................... 54
Ranibizumab (LUCENTIS®) .................................................................................................. 55
Rituximab (MABTHERA®).................................................................................................... 56

4
Siltuximab (SYLVANT®) ...................................................................................................... 57
Tocilizumab (ROACTEMRA®) .............................................................................................. 58
Trastuzumab (HERCEPTIN®)............................................................................................... 59
Trastuzumab emtansine (KADCYLA®)................................................................................. 60
Ustekinumab (STELARA®) ................................................................................................... 61
Vedolizumab (ENTYVIO®) .................................................................................................. 62
CARACTERISTIQUES DES RECEPTEURS SOLUBLES THERAPEUTIQUES .......................................... 63
Abatacept (ORENCIA®) ....................................................................................................... 64
Aflibercept (ZALTRAP®, EYLEA®) ......................................................................................... 65
Anakinra (KINERET®) .......................................................................................................... 66
Belatacept (NULOJIX®) ....................................................................................................... 67
Etanercept (ENBREL®) ........................................................................................................ 68
INDEX ........................................................................................................................................ 69
REFERENCES BIBLIOGRAPHIQUES .............................................................................................. 72
 6
6
 7
7
 8
8
 9
9
 10
10
 11
11
 12
12
 13
13
 14
14
 15
15
 16
16
 17
17
 18
18
 19
19
 20
20
 21
21
 22
22
 23
23
 24
24
 25
25
 26
26
 27
27
 28
28
 29
29
 30
30
 31
31
 32
32
 33
33
 34
34
 35
35
 36
36
 37
37
 38
38
 39
39
 40
40
 41
41
 42
42
 43
43
 44
44
 45
45
 46
46
 47
47
 48
48
 49
49
 50
50
 51
51
 52
52
 53
53
 54
54
 55
55
 56
56
 57
57
 58
58
 59
59
 60
60
 61
61
 62
62
 63
63
 64
64
 65
65
 66
66
 67
67
 68
68
 69
69
 70
70
 71
71
 72
72
 73
73
 74
74
 75
75
 76
76
 77
77
 78
78
 79
79
1
/
79
100%