Ulcazal Leaflet - Arwan Pharmaceutical Industries

Composition
Each vial contains:
Active ingredient: Lyophilized (freeze-dried) omeprazole
sodium equivalent to omeprazole 40 mg.
Excipients: Disodium edetate and sodium hydroxide.
Properties
Ulcazal represents the current trend in the treatment of
peptic ulcer and allied conditions whereby the final step of
gastric acid secretion is inhibited irrespective of the
stimulus. Ulcazal acts specifically by inhibiting the H+/K+
ATPase enzyme system (the 'proton pump') at the
secretory surface of the gastric parietal cells, blocking
thereby the final transport of hydrogen ions into the gastric
lumen, and thus inhibiting effectively both basal and
stimulated acid secretion.
Ulcazal has also antimicrobial activity against Helicobac-
ter pylori by selective inhibition of H. pylori urease, which is
necessary for gastric colonization.
Ulcazal has no effect on acetylcholine or histamine
receptors.
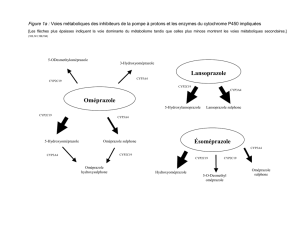
After intravenous administration, omeprazole distributes
rapidly to extravascular sites. The volume of distribution of
omeprazole may be slightly decreased in elderly and in
patients with hepatic insufficiency but it is not markedly
affected in patients with renal impairment. The elimination
half-life of omeprazole from plasma is short, being
reported to be about 0.5-3 h, however, its duration of action
with regard to inhibition of acid secretion is much longer
allowing it to be used in single daily doses. Omeprazole is
highly bound (about 95%) to plasma protein. It is almost
completely metabolized in the liver, primarily by the
cytochrome P450 system. Almost 80% of an intravenously
given dose is excreted as metabolites in the urine and the
remainder is found in the faeces, primarily originating from
bile secretion.
Indications
Duodenal ulcer, gastric ulcer and reflux oesophagitis.
Zollinger-Ellison syndrome.
Oméprazole sodique
Poudre lyophilisée stérile pour solution pour
injection I.V. ou perfusion
Composition
Chaque flacon contient:
Substance active: Oméprazole de sodium lyophilisé
(desséché
par congélation) équivalent à 40 mg d’oméprazole.
Excipients: Édétate disodique et hydroxyde de sodium.
Propriétés
Ulcazal représente actuellement l’approche convention-
nelle dans le traitement de l’ulcère peptique et des
conditions associées, qui consiste en l’inhibition de l’étape
finale de la sécrétion d’acide gastrique et ceci indépendam-
ment du stimulus. Ulcazal agit spécifiquement en inhibant le
système H+/K+ ATPase (pompe à protons) à la surface
sécrétoire des cellules pariétales gastriques, bloquant ainsi
le transport final des ions hydrogène vers la lumière
gastrique, inhibant effectivement à la fois la sécrétion acide
basale et stimulée.
Ulcazal a aussi une activité antimicrobienne contre
l’hélicobacter pylori par inhibition sélective de l'uréase
produite par l’hélicobacter pylori et nécessaire à la
colonisation gastrique.
Ulcazal n’a pas d’effet sur les récepteurs de l’acétylcholine
ou de l’histamine.
Après administration intraveineuse, l’oméprazole se distribue
rapidement vers les sites extravasculaires. Le volume de
distribution d’oméprazole pourrait être légèrement réduit
chez les patients âgés et chez ceux souffrant d’insuffisance
hépatique, mais n’est pas significativement affecté chez les
patients souffrant de troubles de la fonction rénale. La
demi-vie d’élimination plasmatique de l’oméprazole est
courte, entre 0,5 et 3 h; cependant, sa durée d’action quant à
l’inhibition de la sécrétion d’acide est bien plus longue,
permettant son utilisation à des doses quotidiennes uniques.
L’oméprazole est fortement lié (95% environ) aux protéines
plasmatiques. Il est presque entièrement métabolisé dans le
foie, essentiellement par le système cytochrome P450.
Environ 80% d’une dose intraveineuse donnée est excrétée
sous forme de métabolites dans l’urine et le reste est
retrouvé dans les fèces, provenant principalement de la
sécrétion biliaire.
Indications
Ulcère gastroduodénal, œsophagite par reflux
gastro-œsophagien, syndrome de Zollinger-Ellison.
Thus, upon concurrent administration, omeprazole may
result in delayed elimination, increased blood concentra-
tions, and enhanced effects of these medications.
Monitoring of blood concentrations, or prothrombin time for
warfarin, is recommended as a guide of dosage since
dosage adjustment of these medications may be
necessary during and after omeprazole therapy.
Absorption of ketoconazole and itraconazole may be
reduced upon concurrent administration of omeprazole.
Plasma concentration of digoxin and tacrolimus may
possibly be increased upon concomitant administration of
omeprazole.
Presentation
Ulcazal sterile lyophilized powder for solution for I.V.
injection or infusion is available in packs of 1 or 10 vials
containing 40 mg omeprazole.
Storage conditions
Store below 25°C. Protect from light.
Manufacturer and MAH:
ARWAN Pharmaceutical Industries,
Jadra, Lebanon
Omeprazole sodium
Sterile lyophilized powder for solution for I.V. injection or infusion
THIS IS A MEDICAMENT
Medicament is a product which affects your health, and its
consumption contrary to instructions is dangerous for you.
Follow strictly the doctor's prescription, the method of use and
the instructions of the pharmacist who sold the medicament.
The doctor and the pharmacist are experts in medicines,
their benefits and risks.
Do not by yourself interrupt the period of treatment
prescribed for you.
Do not repeat the same prescription without consulting
your doctor.
Keep all medicaments out of the reach of children.
•
•
•
•
•
•
Council of Arab Health Ministers
Union of Arab Pharmacists
Reconstitution and administration
Injection: the solution for IV injection is obtained by adding
10 ml water for injection to the vial containing the lyophilized
powder. The reconstituted solution of Ulcazal should be
given slowly over a period of 2 to 5 minutes at a maximum
rate of 4ml/minute.
After reconstitution, the solution should be administered
immediately.
Infusion: The entire contents of each vial is to be dissolved
in approximately 5 ml and then immediately diluted to 100
ml. Physiological Sodium chloride (0.9%) solution for
infusion or glucose (5%) solution for infusion must be used.
The reconstituted solution of Ulcazal should be given as an
intravenous infusion over a period of 20- 30 minutes.
Ulcazal is stable for 12 hours when reconstituted with 0.9%
sodium chloride and for 6 hours when reconstituted with 5%
glucose solution at 25 °C.
From a microbiological point of view, the product should be
used immediately unless it has been reconstituted under
controlled and validated aseptic conditions.
Contraindications
Ulcazal is contraindicated in individuals with known
hypersensitivity to omeprazole.
Omeprazole, like other proton pump inhibitors, should not
be co-administered with atazanavir (see Interactions).
Like other proton pump inhibitors, omeprazole is better to be
avoided (unless it is considered essential) during pregnancy
and lactation until further research studies are available.
Precautions
As general practice in treating gastric ulcer, the possibility of
gastric malignancy should be excluded prior to the initiation
of therapy with omeprazole. Omeprazole may mask
symptoms of gastric cancer; particular care is required in
those whose symptoms change and in those over 45 years
of age.
Impaired hepatic functions: As omeprazole is extensively
metabolized in the liver, its plasma half-life is increased in
patients having hepatic impairment; caution is
recommended in such patients and the recommended daily
dose should not exceed 20 mg.
Impaired renal functions: Dosage adjustment is not
needed in patients with impaired renal functions.
Geriatrics: Dosage adjustment is not needed in geriatric
patients.
Pediatrics: As there is limited experience with the use of
omeprazole in pediatric age group, its use is not
recommended in children.
Side effects
Omeprazole is generally well tolerated. Only transient and
reversible side effects have been reported.
The following side effects, listed by body system, have been
reported with the use of omeprazole, which is more or less
similar to that associated with other proton pump inhibitors:
Nervous system: Headache, dizziness, vertigo, blurred
vision, visual impairment (with high doses), taste
disturbances, insomnia, somnolence, depression, and
paraesthesia. Reversible confusional states, agitation, and
hallucinations have been reported in severely ill patients.
Gastrointestinal tract: Dry mouth, stomatitis, nausea,
vomiting, flatulence, abdominal pain, diarrhea, and
constipation.
Like all other proton pump inhibitors, omeprazole decreases
gastric acidity and may increase the risk of gastrointestinal
infections.
Liver: Rarely; transient disturbances in liver enzymes. In
isolated cases; hepatic dysfunction, hepatitis with or without
jaundice, and encephalopathy in patients with severe liver
disease.
Kidney: Interstitial nephritis.
Musculoskeletal system: Malaise, muscle and joint pain,
fracture of the hip, wrist or spine.
Skin: In isolated cases; pruritus, photosensitivity, erythema
multiforme, alopecia, bullous eruption, Stevens-Johnson
syndrome, toxic epidermal necrolysis, and anaphylaxis.
Hypersensitivity reactions: Rash, urticaria, angioedema,
and bronchospasm.
Hematological: Agranulocytosis, leucopenia, pancytope-
nia, and thrombocytopenia.
Others: Fever, sweating, hyponatremia, hypomagnesae-
mia, peripheral edema, gynecoamastia, and rarely
impotence.
Overdosage
It has been reported that intravenous administration of
omeprazole in doses up to 270 mg on a single day and up to
650 mg over a 3-day period has not resulted in any
dose-related adverse reactions.
Drug interactions
Omeprazole may reduce the hepatic metabolism of
warfarin, diazepam, phenytoin, and possibly other drugs
metabolized via cytochrome P450 enzyme system.
Dosage
Duodenal ulcer,gastric ulcer and reflux oesophagitis:
Patients who cannot be given oral medication can be treated
parenterally with 40 mg once daily.The usual treatment
period before transfer to oral treatment is 2-3 days.
In Zollinger-Ellison syndrome: the dose should be
adjusted individually. Higher doses and/or several doses
daily may be required.
Intravenous treatment can be given as an infusion over a
period of 20-30 minutes. After reconstitution start the
infusion immediately.
Impaired renal function
A dose adjustment is not necessary for patients with
impaired renal function.
Impaired liver function
In patients with impaired liver function clearance is greatly
reduced.
Elderly patients
A dose adjustment is not necessary in elderly patients.
Children
There is only limited experience of treatment in children.
Reconstitution et administration
Injection: la solution pour injection I.V. est obtenue par
addition de 10 ml d’eau pour préparation injectable au
flacon contenant la poudre lyophilisée. La solution obtenue
doit être administrée par injection lente sur une période de
2 à 5 minutes avec une vitesse maximale de 4ml/minutes.
Après reconstitution, la solution doit être utilisée immédiate-
ment.
Perfusion: Le contenu de chaque flacon doit être dissous
dans 5 ml puis dilué immédiatement dans 100 ml. Une
solution de chlorure de sodium à 0,9 % ou une glucosé à
5 % doit être utilisée. La solution obtenue doit être
administrée par voie intraveineuse en perfusion sur une
période de 20 – 30 minutes.
Ulcazal est stable pour 12 heures une fois reconstituée
avec de chlorure de sodium 0,9% et pour 6 heures après
reconstitution avec une solution glucosé à 5 % à 25 °C.
D’un point de vue microbiologique, le produit doit ȇtre utilisé
immédiatemment sauf s’il a été reconstitué dans des
conditions aseptiques contrôlées et validées.
Contre-indications
Ulcazal est contre-indiqué chez les individus ayant une
hypersensibilité connue à l’oméprazole.
Oméprazole, comme les autres inhibiteurs de la pompe à
protons, ne doit pas être administré avec l'atazanavir (cf
Interactions).
®
ULCAZAL
®
ULCAZAL
“ULCAZAL” is a trade mark
123029
Dosages
Ulcère gastroduodénal, reflux
gastro-œsophagien/gastrite: chez Les patients qui ne
peuvent pas être traités par voie orale, une doses de 40
mg est recommandée. La durée nécessaire de
traitement est généralement 2-3 jours.
Syndrome de Zollinger-Ellison: la posologie sera
ajustée individuellement, la posologie peut être
augmentée progressivement chez certains patients. La
dose journalière doit être répartie en plusieurs prises en
cas de l’utilisation de dose élevés.
Ulcazal peut être administrer par perfusion intraveineuse
en 20-30 minutes. La solution reconstituée doit être
administrée immédiatement
Insuffisance rénale
L'ajustement de la dose n'est pas nécessaire chez les
patients souffrant d'insuffisance rénale.
Insuffisance hépatique
Chez les patients souffrant d’insuffisance hépatique, une
diminution de la clairance hépatique est observée.
Patients âgés
L'ajustement de la dose n'est pas nécessaire chez les
patients âgés.
Enfants
Les expériences sur le traitement chez l’enfant sont
limitées à ce jour.
LE0001-05
Revision date: May 2008

Comme tous les autres inhibiteurs de la pompe à protons,
il est préférable d’éviter l’oméprazole (sauf s’il est
considéré comme nécessaire) en période de grossesse et
d’allaitement jusqu’à ce que des études et des recherches
plus avancées soient disponibles.
Précautions
Comme pratique générale dans le traitement de l’ulcère
gastrique, il faut d’abord éliminer toute possibilité de
malignité gastrique avant d’entamer la thérapie avec
l’oméprazole. L’oméprazole pourrait masquer les
symptômes de cancer gastrique, il faut donc accorder des
soins particuliers aux patients présentant une symptoma-
tologie variable et à ceux qui ont plus de 45 ans.
Fonctions hépatiques perturbées: Comme l’oméprazole
est extensivement métabolisé dans le foie, sa demi-vie
plasmatique est augmentée chez les patients ayant des
troubles hépatiques; pour cela il est recommandé de
prendre des précautions chez de tels patients et la dose
quotidienne recommandée ne doit pas dépasser 20 mg.
Fonctions rénales perturbées: Pas besoin d’ajustement
de dose chez les patients ayant des troubles de la fonction
rénale.
Gériatrie: Pas besoin d’ajustement de dose chez les
patients gériatriques.
Pédiatrie: Vue l’expérience limitée, l’usage de l’oméprazole
n’est pas recommandé chez les enfants.
Effets secondaires
L’oméprazole est généralement bien toléré. Seul des effets
secondaires transitoires et réversibles ont été rapportés.
Les effets secondaires suivants, classés par système
fonctionnel, ont été associés à l’usage de l’oméprazole et
sont plus ou moins similaires à ceux associés à d’autres
inhibiteurs de la pompe à protons.
Système nerveux: Maux de tête, étourdissements,
vertiges, vue trouble, altération de la vue (avec les doses
élevées), troubles du goût, insomnie, somnolence,
dépression et paresthésie. Des états réversibles de
confusion, d’agitation et d’hallucination ont été rapportés
chez des patients gravement malades.
Appareil gastro-intestinal: Sécheresse de la bouche,
stomatite, nausée, vomissements, flatulence, douleur
abdominale, diarrhée et constipation.
Comme tous les autres inhibiteurs de la pompe à protons,
l’oméprazole réduit l’acidité gastrique et pourrait
augmenter le risque d’infections gastro-intestinales.
Foie: Rarement, des perturbations transitoires des
enzymes hépatiques. Dans des cas isolés, dysfonctionne-
ment hépatique, hépatite, avec ou sans jaunisse, et
encéphalopathie chez les patients souffrant d’une atteinte
hépatique sévère.
Rein: Néphrite interstitielle.
Système Musculosquelettiques: Malaise, douleurs
musculaires et articulaires, fracture de la hanche, poignet
ou des vertèbres.
Peau: Dans des cas isolés; prurit, photosensibilité,
érythème multiforme, alopécie, éruption bulleuse, syndrome
de Stevens-Johnson, nécrose épidermique toxique et
anaphylaxie.
Réactions d’hypersensibilité: Eruption cutanée, urticaire,
angiœdème et bronchospasme.
Hématologiques: Agranulocytose, leucopénie, pancytopénie
et thrombocytopénie.
Autres: Fièvre, sudation, hyponatrémie, hypomagnésémie,
œdème périphérique, gynécomastie et des cas rares
d’impuissance.
Surdosage
Aucune réaction indésirable, liée à la dose, n’a été observée
suite à l’administration intraveineuse de l’oméprazole à des
doses allant jusqu’à 270 mg en un seul jour et jusqu’à 650
mg sur une période de 3 jours.
Interactions médicamenteuses
L’oméprazole pourrait réduire le métabolisme hépatique
de la warfarine, du diazépam, de la phénytoïne, et
peut-être d’autres médicaments métabolisés par les
enzymes du système cytochrome P450. Par suite, lors
d’une administration concomitante, l’oméprazole pourrait
retarder l’élimination de ces médicaments et aboutir à une
augmentation de leurs concentrations dans le sang et à
une potentialisation de leurs effets. Pour cela, il est
recommandé, durant et après le traitement oméprazole,
de surveiller les concentrations sanguines, ou le temps de
prothrombine pour la warfarine, comme guide pour la
posologie de ces médicaments, puisqu’ un ajustement de
dose pourrait être nécessaire.
L’absorption du kétoconazole et de l’itraconazole pourrait
être réduite lors de l’administration concomitante
d’oméprazole.
La concentration de digoxine et de tacrolimus dans le
plasma pourrait être augmentée lors de l’administration
concomitante d’oméprazole.
Présentation
Ulcazal poudre lyophilisée stérile pour solution pour
injection I.V. ou perfusion est disponible en boîtes
contenant 1 ou 10 flacons de 40 mg d’oméprazole.
Conditions de conservation
À conserver à une température inférieure à 25°C, à l’abri
de la lumière.
Fabricant et Titulaire de l’autorisation de mise sur
le marché :
ARWAN Pharmaceutical Industries, Jadra, Liban
ﺀﺎﻄﻋﻹﺍﻭ ﺮﻴﻀﺤﺘﻟﺍ
ءﺎﻣﺭﺗﻠﻳﻠﻠﻣ ۱۰ ﺔﻓﺎﺿﺇ ﺭﺑﻋ ﺩﻳﺭﻭﻟﺍ ﻲﻓ ﻥﻘﺣﻠﻟ ﻝﻭﻠﺣﻣﻟﺍ ﺭّﺿﺣﻳ :ﻥﻘﺣﻟﺍ
ﺭّﺿﺣﻣﻟﺍ ﻝﻭﻠﺣﻣﻟﺍ ﻰﻁﻌﻳ . .ﺩﻳﻣﺟﺗﻟﺎﺑ ﻑﻔﺟﻣﻟﺍ ﻕﻭﺣﺳﻣﻟﺍ ﺓﻭﺑﻋ ﻲﻓ ﻥﻘﺣﻠﻟ
ﻥﻳﺗﻘﻳﻗﺩﻟﺍ ﻥﻳﺑ ﺡﻭﺍﺭﺗﺗ ﺓﺭﺗﻓ ﻯﺩﻣ ﻰﻠﻋ ﺩﻳﺭﻭﻟﺍ ﻲﻓ ءﻲﻁﺑﻟﺍ ﻥﻘﺣﻟﺍ ﻕﻳﺭﻁ ﻥﻋ
.ﺔﻘﻳﻗﺩ/ﻝﻠﻣ ٤ ﻯﻭﺻﻗ ﺔﻋﺭﺳ ﻊﻣ ﻕﺋﺎﻗﺩ ﺔﺳﻣﺧﻟﺍﻭ
.ﺭﻭﻔﻟﺍ ﻰﻠﻋ ﻝﻭﻠﺣﻣﻟﺍ ﻡﺍﺩﺧﺗﺳﺇ ﺏﺟﻳ ،ﺭﻳﺿﺣﺗﻟﺍ ﺩﻌﺑ
ﺭﺗﻠﻳﻠﻠﻣ ۱۰ ﻥﻘﺣ ﻕﻳﺭﻁ ﻥﻋ ّﻱﺩﻳﺭﻭﻟﺍ ﻝﻭﻠﺣﻣﻟﺍ ﺭّﺿﺣﻳ :ﻱﺩﻳﺭﻭﻟﺍ ّﻱﺭﻟﺍ
ﻑﻔﺟﻣﻟﺍ ﻕﻭﺣﺳﻣﻟﺍ ﺓﻭﺑﻋ ﻲﻓ ٪۰,۹ ﻡﻭﻳﺩﻭﺻﻟﺍ ﺩﻳﺍﺭﻭﻠﻛ ﻝﻭﻠﺣﻣ ﻥﻣ
ﺯﻳﻛﺭﺗﻟﺍ ﻑﻳﻔﺧﺗ ﺩﻌﺑ ّﻱﺭﻟﺍ ﻕﻳﺭﻁ ﻥﻋ ﺭّﺿﺣﻣﻟﺍ ﻝﻭﻠﺣﻣﻟﺍ ﻰﻁﻌﻳ .ﺩﻳﻣﺟﺗﻟﺎﺑ
.٪٥ ﺯﻭﻛﻭﻠﻐﻟﺍ ﻝﻭﻠﺣﻣ ﻭﺃ ٪۰,۹ ﻲﺣﻠﻣﻟﺍ ﻝﻭﻠﺣﻣﻟﺍ ﻥﻣ ﺭﺗﻠﻳﻠﻠﻣ ۱۰۰ ﻲﻓ
"ﺎﺗﺑﺎﺛ ﻝﺍﺯﺎﺳﻟﺃ ﻰﻘﺑﻳ ،٪۰,۹ ﻡﻭﻳﺩﻭﺻﻟﺍ ﺩﻳﺍﺭﻭﻠﻛ ﻝﻭﻠﺣﻣ ﻊﻣ ﺭﻳﺿﺣﺗﻟﺍ ﺩﻌﺑ
ﺩﻧﻋ ٪٥ﺯﻭﻛﻭﻠﻏ ﻝﻭﻠﺣﻣ ﻊﻣ ﻩﺭﻳﺿﺣﺗ ﺩﻧﻋ ﺕﺎﻋﺎﺳ ٦ ﺓﺩﻣﻟﻭ ﺔﻋﺎﺳ۱۲ﺓﺩﻣﻟ
.ﻡ°۲٥ ﺔﺟﺭﺩ
ﻡﺗ ﺍﺫﺍ ﻻﺇ ﺓﺭﺷﺎﺑﻣ ءﺍﻭﺩﻟﺍ ﻡﺍﺩﺧﺗﺳﺇ ﺏﺟﻳ ،ﺔﻳﺟﻭﻟﻭﻳﺑﻭﺭﻛﻳﻣﻟﺍ ﺔﻳﺣﺎﻧﻟﺍ ﻥﻣ
.ﺔﻣﻛﺣﻣ ﺔﻣﻳﻘﻋ ﻁﻭﺭﺷﺑ ﻩﺭﻳﺿﺣﺗ
ﻝﺎﻣﻌﺗﺳﻹﺍ ﻲﻫﺍﻭﻧ
ﺔﻁﺭﻔﻣﻟﺍ ﺔﻳﺳﺎﺳﺣﻟﺍ ﻱﻭﺫ ﻰﺿﺭﻣﻟﺍ ﻝﺑﻗ ﻥﻣ ﻝﺍﺯﺎﺳﻟﺃ ﻝﺎﻣﻌﺗﺳﺍ ﻡﺩﻋ ﺏﺟﻳ
.ﻝﻭﺯﺍﺭﺑﻳﻣﻭﻸﻟ
ﻥﻭﺗﻭﺭﺑﻟﺍ ﺔّﺧﺿﻣ ﺕﺎﻁّﺑﺛﻣ ﻥﻣ ﻱﺃ ﻭﺃ) ﻝﻭﺯﺍﺭﺑﻳﻣﻭﻷﺍ ءﺎﻁﻋﺇ ﺏﺟﻳ ﻻ
.(ﺔّﻳﺋﺍﻭﺩﻟﺍ ﺕﻼﻋﺎﻔﺗﻟﺍ ﺔﻌﺟﺍﺭﻣ ﻰﺟﺭﻳ) ﺭﻳﻓﺎﻧﺯﺎﺗﻷﺍ ﻊﻣ (ﻯﺭﺧﻷﺍ
ﺏﻧﺟﺗ ﻝﺿﻔﻳ ﻪﻧﺈﻓ ،ﻯﺭﺧﻷﺍ ﻥﻭﺗﻭﺭﺑﻟﺍ ﺔﺧﺿﻣ ﺕﺎﻁﺑﺛﻣ ﻊﻣ ﻝﺎﺣﻟﺍ ﻭﻫ ﺎﻣﻛ
ﻙﻟﺫ ﺩﻌﻳ ﻡﻟ ﺎﻣ) ﺔﻋﺎﺿﺭﻟﺍﻭ ﻝﻣﺣﻟﺍ ﻲﺗﺭﺗﻓ ﻝﻼﺧ ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ﻡﺍﺩﺧﺗﺳﺍ
.ﻙﻟﺫ ﻝﻭﺣ ﺙﻭﺣﺑﻟﺍﻭ ﺕﺎﺳﺍﺭﺩﻟﺍ ﻥﻣ ﺩﻳﺯﻣﻟﺍ ﺭﻓﻭﺗ ﻥﻳﺣ ﻰﻟﺇ (ًﺎﻳﺭﻭﺭﺿ
ﺕﺎﻁﺎﻳﺗﺣﻹﺍ
ﻡﺍﺭﻭﺃ ﺩﻭﺟﻭ ﻡﺩﻋ ﻥﻣ ﺩﻛﺄﺗﻟﺍ ﺏﺟﻳ ،ﺓﺩﻌﻣﻟﺍ ﺔﺣﺭﻗ ﺝﻼﻋ ﺩﻧﻋ ﺔﻣﺎﻋ ﺓﺩﻋﺎﻘﻛ
ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ﻲﻔﺧﻳ ﺩﻗ ﺫﺇ ،ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺄﺑ ﺝﻼﻌﻟﺍ ءﺩﺑ ﻝﺑﻗ ﺓﺩﻌﻣﻟﺎﺑ ﺔﺛﻳﺑﺧ
ﻥﻳﺫﻟﺍ ﻰﺿﺭﻣﻠﻟ ﺔﺻﺎﺧﻟﺍ ﺔﻳﺎﻧﻌﻟﺍ ﺭﻳﻓﻭﺗﺑ ﻰﺻﻭﻳ .ﺓﺩﻌﻣﻟﺍ ﻥﺎﻁﺭﺳ ﺽﺍﺭﻋﺃ
ﻡﻫﺭﺎﻣﻋﺃ ﺩﻳﺯﺗ ﻥﻳﺫﻠﻟ ﻙﻟﺫﻛﻭ ﺓﺩﻌﻣﻟﺍ ﺔﺣﺭﻗ ﺽﺍﺭﻋﺃ ﺭﻳﻐﺗ ﻥﻣ ﻥﻭﻛﺷﻳ ﺩﻗ
.ﺔﻧﺳ ٤٥ ﻥﻋ
ﺩﺑﻛﻟﺍ ﻲﻓ ﺓﺭﻳﺑﻛ ﺔﺟﺭﺩ ﻰﻟﺇ ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ﺽﻳﺅﻳ :ﺪﺒﻜﻟﺍ ﻒﺋﺎﻇﻭ ﺭﻮﺼﻗ
ﻥﻣ ﻥﻭﻧﺎﻌﻳ ﻥﻳﺫﻟﺍ ﻰﺿﺭﻣﻟﺍ ﻯﺩﻟ ﻲﻔﺻﻧﻟﺍ ﺭﻣﻌﻟﺍ ﺓﺩﺎﻳﺯ ﻙﻟﺫ ﻥﻋ ﺞﺗﻧﻳﻭ
ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ءﺎﻁﻋﺇ ﺩﻧﻋ ﺭﺫﺣﻟﺎﺑ ﻰﺻﻭﻳ ﺍﺫﻟ ،ﺩﺑﻛﻟﺍ ﻑﺋﺎﻅﻭ ﻲﻓ ﺭﻭﺻﻗ
ﻲﻓ ﻡﻐﻠﻣ ۲۰ ﺔﻓﻭﺻﻭﻣﻟﺍ ﺔﻋﺭﺟﻟﺍ ﺯﻭﺎﺟﺗﺗ ﻻﺃ ﺏﺟﻳ ﺎﻣﻛ ،ﻰﺿﺭﻣﻟﺍ ءﻻﺅﻬﻟ
.ﻡﻭﻳﻟﺍ
ﺔﻋﺭﺟﻟﺍ ﻰﻠﻋ ﻝﻳﺩﻌﺗ ﻱﺃ ءﺍﺭﺟﺇ ﺭﻣﻷﺍ ﺏﺟﻭﺗﺳﻳ ﻻ :ﻰﻠﻜﻟﺍ ﻒﺋﺎﻇﻭ ﺭﻮﺼﻗ
.ﻰﻠﻛﻟﺍ ﻑﺋﺎﻅﻭ ﻲﻓ ﺭﻭﺻﻗ ﻥﻣ ﻥﻭﻧﺎﻌﻳ ﻥﻳﺫﻟﺍ ﻰﺿﺭﻣﻟﺍ ﺔﻟﺎﺣ ﻲﻓ
ءﻻﺅﻫ ﺩﻧﻋ ﺔﻋﺭﺟﻟﺍ ﻰﻠﻋ ﻝﻳﺩﻌﺗ ﻱﺃ ءﺍﺭﺟﺇ ﺭﻣﻷﺍ ﺏﺟﻭﺗﺳﻳ ﻻ :ﻦﺴﻟﺍ ﺭﺎﺒﻛ
.ﻰﺿﺭﻣﻟﺍ
ﺭﻓﻭﺗ ﺔﻠﻘﻟ ﻙﻟﺫﻭ ﻝﺎﻔﻁﻸﻟ ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ﻡﺍﺩﺧﺗﺳﺎﺑ ﻰﺻﻭﻳ ﻻ :ﻝﺎﻔﻃﻷﺍ
.ﻝﺎﻔﻁﻷﺍ ﻯﺩﻟ ﻪﻣﺍﺩﺧﺗﺳﺍ ﻥﺎﻣﺃ ﻯﺩﻣ ﻥﻋ ﺕﺎﻣﻭﻠﻌﻣﻟﺍﻭ ﺏﺭﺎﺟﺗﻟﺍ
ﺔﻳﺑﻧﺎﺟﻟﺍ ﺕﺍﺭﻳﺛﺄﺗﻟﺍ
ﺎﻬﻠﻳﺟﺳﺗ ﻡﺗ ﻲﺗﻟﺍ ﺔﻳﺑﻧﺎﺟﻟﺍ ﺕﺍﺭﻳﺛﺄﺗﻟﺍ ﺩﻌﺗﻭ ،ﺓﺩﺎﻋ ﻝّﻣﺣﺗﻟﺍ ﺩﻳﺟ ﻝﻭﺯﺍﺭﺑﻳﻣﺃ ﻥﺇ
.ءﺍﻭﺩﻟﺍ ﻑﺎﻘﻳﺇ ﺩﺭﺟﻣﺑ ﻝﻭﺯﺗﻭ ﺓﺭﺑﺎﻋ
ﺎﻬﻠﻳﺟﺳﺗ ﻡﺗ ﻡﺳﺟﻟﺍ ﺓﺯﻬﺟﻷ ًﺎﻘﻓﻭ ﺔﻔﻧﺻﻣﻟﺍﻭ ﻩﺎﻧﺩﺃ ﺓﺭﻭﻛﺫﻣﻟﺍ ﺔﻳﺑﻧﺎﺟﻟﺍ ﺕﺍﺭﻳﺛﺄﺗﻟﺍ ﻥﺇ
ﺔﺟﺗﺎﻧﻟﺍ ﺔﻳﺑﻧﺎﺟﻟﺍ ﺕﺍﺭﻳﺛﺄﺗﻠﻟ ﺔﻠﺛﺎﻣﻣ ﺩﻌﺗ ﺎﻣ ًﺎﺑﻟﺎﻏ ﻲﺗﻟﺍﻭ ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ﻝﺎﻣﻌﺗﺳﺍ ﺩﻧﻋ
:ﻲﻫﻭ ،ﻥﻭﺗﻭﺭﺑﻟﺍ ﺔﺧﺿﻣﻟ ﺔﻁﺑﺛﻣﻟﺍ ﺔﻳﻭﺩﻷﺍ ﻥﻣ ﻱﺃ ﻝﺎﻣﻌﺗﺳﺍ ﻥﻋ
ﻲﻓ ﻑﻌﺿ ،ﺔﻳﺅﺭﻟﺍ ﺡﻭﺿﻭ ﻡﺩﻋ ،ﺭﺍﻭﺩ ،ﺔﺧﻭﺩ ،ﻉﺍﺩﺻ :ﻲﺒﺼﻌﻟﺍ ﺯﺎﻬﳉﺍ
،ﻕﺭﺃ ،ﻕﻭﺫﺗﻟﺍ ﻲﻓ ﺕﺎﺑﺍﺭﻁﺿﺍ ،(ﺓﺭﻳﺑﻛ ﺕﺎﻋﺭﺟ ﻝﺎﻣﻌﺗﺳﺍ ﺩﻧﻋ) ﺭﺻﺑﻟﺍ
ﻙﺎﺑﺗﺭﻻﺍ ﻥﻣ ﺕﻻﺎﺣ ﺙﻭﺩﺣ ﺎﻧﺎﻳﺣﺃ ﻝﺟﺳ ﺩﻘﻟ .ﺭﺩﺧﻭ ﺏﺎﺋﺗﻛﺍ ،ﺱﺎﻌﻧ
.ﻝﻼﺗﻋﻻﺍ ﻱﺩﻳﺩﺷ ﻰﺿﺭﻣﻟﺍ ﻯﺩﻟ ﺔﺳﻭﻠﻫﻭ ﺞﻳﻬﺗ ﻰﻟﺇ ﺔﻓﺎﺿﺇ ﺱﻛﻌﻧﻣﻟﺍ
ﻲﻓ ﻡﻟﺃ ،ﺥﺎﻔﺗﻧﺍ ،ءﻲﻗ ،ﻥﺎﻳﺛﻏ ،ﻡﻔﻟﺍ ﺏﺎﻬﺗﻟﺍ ،ﻡﻔﻟﺍ ﻑﺎﻔﺟ :ﻲﻤﻀﻬﻟﺍ ﺯﺎﻬﳉﺍ
.ﻙﺎﺳﻣﺇﻭ ﻝﺎﻬﺳﺇ ،ﻥﻁﺑﻟﺍ
ﻥﺈﻓ ،ﻥﻭﺗﻭﺭﺑﻟﺍ ﺔﺧﺿﻣﻟ ﺔﻁﺑﺛﻣﻟﺍ ﻯﺭﺧﻷﺍ ﺔﻳﻭﺩﻷﺍ ﺔﻳﻘﺑ ﻊﻣ ﻝﺎﺣﻟﺍ ﻭﻫ ﺎﻣﻛ
ﻥﻣ ﺩﻳﺯﻳ ﺩﻗ ﻭﻬﻓ ﻲﻟﺎﺗﻟﺎﺑﻭ ﺓﺩﻌﻣﻟﺍ ﺔﺿﻭﻣﺣ ﺔﺟﺭﺩ ﻥﻣ ﻝﻠﻘﻳ ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ
.ﻲﻣﺿﻬﻟﺍ ﺯﺎﻬﺟﻟﺍ ﻲﻓ ﻯﻭﺩﻋ ﺙﻭﺩﺣﺭﻁﺧ
ﻲﻓ ﻝﺟﺳ ﺩﻘﻟﻭ ،ﺩﺑﻛﻟﺍ ﺕﺎﻣﻳﺯﻧﺇ ﻲﻓ ﺓﺭﺑﺎﻋ ﺕﺎﺑﺍﺭﻁﺿﺍ ًﺍﺭﺩﺎﻧ ﺙﺩﺣﺗ ﺩﻗ :ﺪﺒﻜﻟﺍ
ﺙﻭﺩﺣ ﻥﻭﺩﺑ ﻭﺃ ﻊﻣ ﺩﺑﻛﻟﺍ ﺏﺎﻬﺗﻟﺍ ،ﺩﺑﻛﻟﺍ ﻑﺋﺎﻅﻭ ﻲﻓ ﻝﻠﺧ ﺙﻭﺩﺣ ﺔﻠﻳﻠﻗ ﺕﻻﺎﺣ
.ﺩﺑﻛﻟﺍ ﻲﻓ ﺩﻳﺩﺷ ﺽﺭﻣﺑ ﻥﻳﺑﺎﺻﻣﻟﺍ ﻰﺿﺭﻣﻟﺍ ﻯﺩﻟ ﻲﻏﺎﻣﺩ ﻝﻼﺗﻋﺍﻭ ﻥﺎﻗﺭﻳ
.ﻲﻟﻼﺧﻟﺍ ﺔﻳﻠﻛﻟﺍ ﺏﺎﻬﺗﻟﺍ :ﻰﻠﻜﻟﺍ
ﻲﻓ ﺭﺳﻛ ،ﻝﺻﺎﻔﻣﻟﺍﻭ ﺕﻼﺿﻌﻟﺍ ﻲﻓ ﻡﻟﺃﻭ ﻥﻫﻭ :ﺕﻼﻀﻌﻟﺍﻭ ﻡﺎﻈﻌﻟﺍ
.ﻱﺭﻘﻔﻟﺍ ﺩﻭﻣﻌﻟﺍ ﻭﺃ ﻥﻳﻣﺻﻌﻣﻟﺍ ،ﻥﻳﻛﺭﻭﻟﺍ
ﻲﻣﺎﻣﺣﻟﺍ ،ءﻭﺿﻠﻟ ﺱﺳﺣﺗ ،ﺔﻛﺣ ﺙﻭﺩﺣ ﺔﻠﻳﻠﻗ ﺕﻻﺎﺣ ﻲﻓ ﻝﺟﺳ ﺩﻘﻟ :ﺪﻠﳉﺍ
- ﺱﻧﻔﻳﺗﺳ ﺔﻣﺯﻼﺗﻣ ،ﻲﻋﺎﻘﻓ ﺢﻔﻁ ،(ﻊﻠﺻﻟﺍ)ﺭﻌﺷﻟﺍ ﻁﻗﺎﺳﺗ ،ﺔﻠﻛﺷﺗﻣﻟﺍ
.ﻱﺭﺧﻧﺗﻟﺍ ﺓﺭﺷﺑﻟﺍ ﻡﻣﺳﺗ ،ﻥﻭﺳﻧﻭﺟ
ﺏﻌﺷﻟﺍ ﺹﻠﻘﺗﻭ ﺔﻳﺋﺎﻋﻭ ﺔﻣﺫﻭ ،ﻯﺭﺷ ،ﻱﺩﻠﺟ ﺢﻔﻁ :ﺔﻴﺳﺎﺴﳊﺍ ﺕﻼﻋﺎﻔﺗ
.ﺔﻳﺋﺍﻭﻬﻟﺍ
ﺔﻳﻭﻣﺩﻟﺍ ﺕﺎﻳﺭﻛﻟﺍ ﺹﻘﻧ ،ﺔﺑﺑﺣﻣﻟﺃ ﺔﻳﻭﻣﺩﻟﺍ ﺕﺎﻳﺭﻛﻟﺍ ﺹﻘﻧ :ﻡﺪﻟﺍ ﺕﺎﺻﻮﺤﻓ
.ﺔﻳﻭﻣﺩﻟﺍ ﺢﺋﺎﻔﺻﻟﺍ ﺹﻘﻧﻭ ﺔﻳﻭﻣﺩﻟﺍ ﺎﻳﻼﺧﻟﺍ ﺹﻘﻧ ،ءﺎﺿﻳﺑﻟﺍ
ﻲﻓ ﻡﻭﻳﺳﻳﻧﻐﻣﻟﺍ ﻭ ﻡﻭﻳﺩﻭﺻﻟﺍ ﺔﺑﺳﻧ ﺽﺎﻔﺧﻧﺍ ،ﻕﺭﻌﺗ ،ﻰﻣﺣ :ﻯﺮﺧﺃ ﺕﺍﺮﻴﺛﺄﺗ
ﻝﻠﺧ ًﺍﺭﺩﺎﻧﻭ ﻱﺩﺛﻟﺍ ﻡﺧﺿﺗ ﺭﻭﻛﺫﻟﺍ ﺩﻧﻏ ﺙﺩﺣﻳ ﺩﻗﻭ ،ﺔﻳﻓﺭﻁ ﺔﻣﺫﻭ ،ﻡﺩﻟﺍ
.ﺏﺎﺻﺗﻧﻻﺍ
ﺓﺩﺋﺍﺯﻟﺍ ﺔﻋﺭﺟﻟﺍ
ﺩﻌﺑ ﻰﺗﺣ ﺔﻋﺭﺟﻟﺍ ﺓﺩﺎﻳﺯﺑ ﺔﻘﻠﻌﺗﻣ ﺔﻳﺑﻧﺎﺟ ﺕﺍﺭﻳﺛﺄﺗ ﺔﻳﺃ ﺙﻭﺩﺣ ﻝﺟﺳﻳ ﻡﻟ
ﻲﻓ ﻡﻐﻠﻣ ۲۷۰ﻰﻟﺇ ﻝﺻﺗ ﺕﺎﻋﺭﺟﺑ ﺩﻳﺭﻭﻟﺍ ﻕﻳﺭﻁ ﻥﻋ ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ءﺎﻁﻋﺇ
.ﻡﺎﻳﺃ ۳ ﻯﺩﻣ ﻰﻠﻋ ﻡﻐﻠﻣ ٦٥۰ ﻭﺃ ﻡﻭﻳﻟﺍ
ﺔﻳﺋﺍﻭﺩﻟﺍ ﺕﻼﻋﺎﻔﺗﻟﺍ
،ﻥﻳﺭﻓﺭﺍﻭ ﻥﻣ ﻝﻛﻟ ﻱﺩﺑﻛﻟﺍ ﺽﻳﻷﺍ ﻲﻓ ًﺎﺿﺎﻔﺧﻧﺍ ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ﺏﺑﺳﻳ ﺩﻗ
ﻡﻭﺭﻛﻭﺗﻳﺳ ﻡﻳﺯﻧﺇ ﺔﻁﺳﺍﻭﺑ ﺽﻳﺅﺗ ﻲﺗﻟﺍ ﺔﻳﻭﺩﻷﺍ ﺽﻌﺑﻭ ﻥﻳﻭﺗﻳﻧﻳﻓ ،ﻡﺎﺑﻳﺯﺎﻳﺩ
ﻱﺩﺅﻳ ﺩﻗ ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ﻊﻣ ﻥﻣﺍﺯﺗﻟﺎﺑ ﺔﻳﻭﺩﻷﺍ ﻩﺫﻫ ﻝﻭﺎﻧﺗ ﻥﺈﻓ ﻙﻟﺫﻟﻭ ٤٥۰ ﺏ
.ﺎﻬﺗﺍﺭﻳﺛﺄﺗ ﻲﻓ ﺓﺩﺎﻳﺯ ﻲﻟﺎﺗﻟﺎﺑﻭ ﻡﺩﻟﺍ ﻲﻓ ﺎﻫﺯﻳﻛﺭﺗ ﺓﺩﺎﻳﺯﻭ ﺎﻬﺣﺭﻁ ﺭﻳﺧﺄﺗ ﻰﻟﺇ
ﻥﻣﺯ ﺔﺑﻗﺍﺭﻣﺑ ﻭﺃ ﻡﺩﻟﺍ ﻲﻓ ﺔﻳﻭﺩﻷﺍ ﻩﺫﻫ ﺯﻳﻛﺭﺗ ﺔﺑﻗﺍﺭﻣﺑ ﻰﺻﻭﻳ
CECI EST UN MEDICAMENT
Un médicament est un produit qui affecte votre santé, et
sa consommation contrairement aux instructions est
dangereuse pour vous.
Suivez strictement les prescriptions du médecin, la
méthode d’utilisation et les instructions du pharmacien
qui vous a vendu le médicament.
Le médecin et le pharmacien sont des experts dans les
médicaments, leurs bienfaits et leurs risques.
N’interrompez pas par vous-mêmes la période de
traitement qui vous est prescrite.
Ne répétez pas la même prescription sans consulter
votre médecin.
Garder les médicaments loin de la portée des enfants.
•
•
•
•
•
•
Conseil des Ministres Arabes de la santé
Union des Pharmaciens Arabes
ﻝﺍﺯﺎﺳﻟﺃ
ﻡﻭﻳﺩﻭﺻ ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ
ﻲﻓ ﻥﻘﺣﻠﻟ ﻝﻭﻠﺣﻣﻟ ﺩﻳﻣﺟﺗﻟﺎﺑ ﻑﻔﺟﻣ ﻡﻘﻌﻣ ﻕﻭﺣﺳﻣ
ﻱﺩﻳﺭﻭﻟﺍ ّﻱﺭﻠﻟ ﻭﺃ ﺩﻳﺭﻭﻟﺍ
ﺏﻳﻛﺭﺗﻟﺍ
:ﻰﻠﻋ ﺓﻭﺑﻋ ﻝﻛ ﻱﻭﺗﺣﺗ
ﻝﺩﺎﻌﻳ ﺎﻣﺑ (ﺩﻳﻣﺟﺗﻟﺎﺑ ﻑﻔﺟﻣ) ﻡﻭﻳﺩﻭﺻﻟﺍ ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ :ﺔﻟﺎﻌﻔﻟﺍ ﺓﺩﺎﻣﻟﺍ
.ﻡﻐﻠﻣ ٤۰ ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ
.ﻡﻭﻳﺩﻭﺻﻟﺍ ﺩﻳﺳﻛﻭﺭﺩﻳﻫﻭ ﻡﻭﻳﺩﻭﺻﻟﺍ ﺕﺎﺗﻳﺩﻳﺇ ﻲﺋﺎﻧﺛ :ﻍﺍﻭﺳﻟﺍ
ﺹﺍﻭﺧﻟﺍ
ﺕﻻﺎﺣﻟﺍﻭ ﻲﻣﺿﻬﻟﺍ ﺯﺎﻬﺟﻟﺍ ﺔﺣﺭﻗ ﺝﻼﻌﻟ ﻲﻟﺎﺣﻟﺍ ﻁﻣﻧﻟﺍ ﻝﺍﺯﺎﺳﻟﺃ ﻝﺛﻣﻳ
ﺓﺩﻌﻣﻟﺍ ﺽﻣﺎﺣ ﺯﺍﺭﻓﺇ ﻁﻳﺑﺛﺗ ﻲﻓ ﻡﻛﺣﺗﻟﺍ ﻪﺗﻁﺳﺍﻭﺑ ﻡﺗﻳ ﺙﻳﺣﺑ ﺎﻬﻟ ﺔﺑﺣﺎﺻﻣﻟﺍ
ﻰﻠﻋ ﻝﺍﺯﺎﺳﻟﺃ ﻝﻣﻌﻳ .ﺽﻣﺎﺣﻟﺍ ﺍﺫﻫ ﺯﺍﺭﻓﺇ ﺓﺩﺎﻳﺯﻟ ﺯﻔﺣﻣﻟﺍ ﻥﻋ ﺭﻅﻧﻟﺍ ﺽﻐﺑ
-ﻲﻧﻳﺟﻭﺭﺩﻳﻬﻟﺍ ﺕﺎﻔﺳﻭﻔﻟﺍ ﻲﺛﻼﺛ ﻥﻳﺳﻭﻧﻳﺩﺃ ﻲﻣﻳﺯﻧﻹﺍ ﻡﺎﻅﻧﻠﻟ ﻲﻋﻭﻧﻟﺍ ﻁﻳﺑﺛﺗﻟﺍ
ﺎﻳﻼﺧﻟﺍ ﻲﻓ ﺯﺍﺭﻓﻹﺍ ﺢﻁﺳ ﻯﻭﺗﺳﻣ ﺩﻧﻋ ﻙﻟﺫﻭ (ﻥﻭﺗﻭﺭﺑﻟﺍ ﺔﺧﺿﻣ) ﻲﺳﺎﺗﻭﺑﻟﺍ
ﺔﻳﺋﺎﻬﻧﻟﺍ ﺔﻠﺣﺭﻣﻟﺍ ﻁﺑﺛﻳ ﻝﻭﻌﻔﻣﻟﺍ ﺍﺫﻫ ﻥﺃ ﺎﻣﺑ .ﺓﺩﻌﻣﻠﻟ ﺔﻧﻁﺑﻣﻟﺍ ﺔﻳﺭﺍﺩﺟﻟﺍ
ﺍﺫﻫ ﺯﺍﺭﻓﺇ ﻁﻳﺑﺛﺗ ﻰﻠﻋ ﺔﻳﻟﺎﻌﻔﺑ ﻝﻣﻌﻳ ﻝﺍﺯﺎﺳﻟﺃ ﻥﺈﻓ ،ﻱﺩﻌﻣﻟﺍ ﺽﻣﺎﺣﻟﺍ ﻥﻳﻭﻛﺗﻟ
.ﺯﻳﻔﺣﺗﻟﺍ ﻥﻋ ًﺎﺟﺗﺎﻧ ﻭﺃ ًﺎﻳﺳﺎﺳﺃ ﺯﺍﺭﻓﻹﺃ ﺍﺫﻫ ﻥﺎﻛ ءﺍﻭﺳ ﺽﻣﺎﺣﻟﺍ
ﺔﻓﻭﺭﻌﻣﻟﺍ ﺎﻳﺭﻳﺗﻛﺑﻟﺍ ﺩﺿ ﺎﻳﺭﻳﺗﻛﺑﻠﻟ ﺩﺎﺿﻣﻛ ﻪﺗﻳﻟﺎﻌﻔﺑ ًﺎﺿﻳﺃ ﻝﺍﺯﺎﺳﻟﺃ ﺯﺎﺗﻣﻳ
ﺯﻳﻳﺭﻭﻳﻟﺍ ﻡﻳﺯﻧﻹ ﻲﺋﺎﻘﺗﻧﺍ ﻁﺑﺛﻣﻛ ﻝﻣﻌﻳ ﺙﻳﺣ ﻱﺭﻭﻠﻳﺎﺑ ﺭﺗﻛﺎﺑﻭﻛﻳﻠﻳﻫ ﻡﺳﺎﺑ
.ﺓﺩﻌﻣﻟﺍ ﻲﻓ ﺎﻬﺗﺍﺭﻣﻌﺗﺳﻣ ﻥﻳﻭﻛﺗﻟ ﻱﺭﻭﺭﺿﻟﺍﻭ ﺎﻳﺭﻳﺗﻛﺑﻟﺍ ﻩﺫﻫ ﻪﺟﺗﻧﺗ ﻱﺫﻟﺍ
ﻭﺃ ﻥﻳﻟﻭﻛ ﻝﻳﺗﺳﻷﺍ ﺕﻼﺑﻘﺗﺳﻣ ﻰﻠﻋ ﺭﻳﺛﺄﺗ ﻱﺃ ﻝﺍﺯﺎﺳﻟﺃ ﺭﺿﺣﺗﺳﻣﻟ ﺱﻳﻟ
.ﻥﻳﻣﺎﺗﺳﻬﻟﺍ
ﺝﺭﺎﺧ ﻰﻟﺇ ﺩﻳﺭﻭﻟﺍ ﻕﻳﺭﻁ ﻥﻋ ءﺎﻁﻋﻹﺍ ﺩﻌﺑ ﺔﻋﺭﺳﺑ ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ﺭﺷﺗﻧﻳ
ﻥﺳﻟﺍ ﺭﺎﺑﻛ ﻯﺩﻟ ًﻼﻳﻠﻗ ﺽﻔﺧﻧﻳ ﺩﻗ ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ﺭﺎﺷﺗﻧﺍ ﻡﺟﺣ ﻥﺇ .ﺔﻳﻭﻣﺩﻟﺍ ﺔﻳﻋﻭﻷﺍ
ﻡﺟﺣ ﺭﺛﺄﺗﻳ ﻻ ﺎﻣﻧﻳﺑ ،ﺩﺑﻛﻟﺍ ﻑﺋﺎﻅﻭ ﻲﻓ ﺭﻭﺻﻗ ﻥﻣ ﻥﻭﻧﺎﻌﻳ ﻥﻳﺫﻟﺍ ﻰﺿﺭﻣﻟﺍﻭ
ﻲﻓ ﺭﻭﺻﻗ ﻥﻣ ﻥﻭﻧﺎﻌﻳ ﻥﻳﺫﻟﺍ ﻰﺿﺭﻣﻟﺍ ﺔﻟﺎﺣ ﻲﻓ ﺔﻅﻭﺣﻠﻣ ﺔﺟﺭﺩﺑ ﻩﺭﺎﺷﺗﻧﺍ
ﻥﻳﺑ ﺡﻭﺍﺭﺗﻳﻭ ﺭﻳﺻﻗ ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ﺡﺭﻁﻟ ﻲﻔﺻﻧﻟﺍ ﺭﻣﻌﻟﺍ ﻥﺇ .ﻰﻠﻛﻟﺍ ﻑﺋﺎﻅﻭ
ﺓﺩﻌﻣﻟﺍ ﺽﻣﺎﺣ ﺯﺍﺭﻓﺇ ﻁﻳﺑﺛﺗ ﺔﻧﻣﺿﺗﻣﻟﺍ ﻪﺗﻳﻠﻋﺎﻓ ﺓﺩﻣ ﻥﺃ ﻻﺇ ،ﺕﺎﻋﺎﺳ ۳- ۲/۱
ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ﻁﺑﺗﺭﻳ .ﺓﺩﺣﺍﻭ ﺔﻳﻣﻭﻳ ﺔﻋﺭﺟﻛ ﻪﻟﻭﺎﻧﺗﺑ ﺢﻣﺳﻳ ﺎﻣﻣ ﻝﻭﻁﺃ ﻥﻭﻛﺗ
ﻝﻣﺎﻛ ﻝﻛﺷﺑ ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ﺽﻳﺅﻳ .(٪۹٥ﻲﻟﺍﻭﺣ) ﺎﻣﺯﻼﺑﻟﺍ ﻥﻳﺗﻭﺭﺑﺑ ﺔﻳﻟﺎﻋ ﺔﺑﺳﻧﺑ
ﻥﻣ ٪۸۰ ﺡﺭﻁ ﻡﺗﻳ .٤٥۰ ﺏ ﻡﻭﺭﻛﻭﺗﻳﺳ ﻡﻳﺯﻧﺇ ﻕﻳﺭﻁ ﻥﻋ ﺩﺑﻛﻟﺍ ﻲﻓ ًﺎﺑﻳﺭﻘﺗ
ﺡﺭﻁﻳﻓ ﻲﻗﺎﺑﻟﺍ ﺎﻣﺃ ﺔﻳﺿﻳﺃ ﺞﺗﺍﻭﻧ ﻝﻛﺷ ﻰﻠﻋ ﻝﻭﺑﻟﺍ ﻕﻳﺭﻁ ﻥﻋ ﺓﺎﻁﻌﻣﻟﺍ ﺔﻋﺭﺟﻟﺍ
.ﺓﺭﺍﺭﻣﻟﺍ ﺕﺍﺯﺍﺭﻓﺇ ﻥﻣ ًﻼﺻﺃ ﺄﺷﻧﻳ ﻱﺫﻟﺍﻭ ﺯﺍﺭﺑﻟﺍ ﻕﻳﺭﻁ ﻥﻋ
ﻝﺎﻣﻌﺗﺳﻻﺍ ﻲﻋﺍﻭﺩ
ﺔﻣﺯﻼﺗﻣ .ﻲﺋﻳﺭﻣﻟﺍ ﻉﺎﺟﺗﺭﻻﺍﻭ ﺔﻳﺩﻌﻣﻟﺍ ﺕﺎﺣﺭﻘﺗﻟﺍ, ﺔﻳﺟﻔﻌﻟﺍ ﺕﺎﺣﺭﻘﺗﻟﺍ
.ﻥﻭﺳﻳﻟﺍ ﺭﺟﻧﻳﻟﻭﺯ
®
ءﺍﻭﺩﻟﺍ ﺍﺫﻫ ﻥﺇ
.ﺭﻁﺧﻠﻟ ﻙﺿﺭﻌﻳ ﺕﺎﻣﻳﻠﻌﺗﻠﻟ ًﺎﻓﻼﺧ ﻪﻛﻼﻬﺗﺳﺍﻭ ﻙﺗﺣﺻ ﻰﻠﻋ ﺭﺛﺅﻳ ﺭﺿﺣﺗﺳﻣ ءﺍﻭﺩﻟﺍ
ﺕﺎﻣﻳﻠﻌﺗﻭ ﺎﻬﻳﻠﻋ ﺹﻭﺻﻧﻣﻟﺍ ﻝﺎﻣﻌﺗﺳﻻﺍ ﺔﻘﻳﺭﻁﻭ ﺏﻳﺑﻁﻟﺍ ﺔﻔﺻﻭ ﺔﻗﺩﺑ ﻊﺑﺗﺇ
.ﻙﻟ ﺎﻬﻓﺭﺻ ﻱﺫﻟﺍ ﻲﻟﺩﻳﺻﻟﺍ
.ﻩﺭﺭﺿﻭ ﻪﻌﻔﻧﺑﻭ ءﺍﻭﺩﻟﺎﺑ ﻥﺍﺭﻳﺑﺧﻟﺍ ﺎﻣﻫ ﻲﻟﺩﻳﺻﻟﺍﻭ ﺏﻳﺑﻁﻟﺍ
.ﻙﺳﻔﻧ ءﺎﻘﻠﺗ ﻥﻣ ﻙﻟ ﺓﺩﺩﺣﻣﻟﺍ ﺝﻼﻌﻟﺍ ﺓﺩﻣ ﻊﻁﻘﺗ ﻻ
.ﺏﻳﺑﻁﻟﺍ ﺓﺭﺎﺷﺗﺳﺍ ﻥﻭﺩﺑ ءﺍﻭﺩﻟﺍ ﻑﺭﺻ ﺭﺭﻛﺗ ﻻ
.ﻝﺎﻔﻁﻷﺍ ﻝﻭﺎﻧﺗﻣ ﻲﻓ ﺔﻳﻭﺩﻷﺍ ﻙﺭﺗﺗ ﻻ
•
•
•
•
•
•
ﺏﺭﻌﻟﺍ ﺔﻟﺩﺎﻳﺻﻟﺍ ﺩﺎﺣﺗﺇ ،ﺏﺭﻌﻟﺍ ﺔﺣﺻﻟﺍ ءﺍﺭﺯﻭ ﺱﻠﺟﻣ
ﺔﻋﺭﺟﻟﺍ
ﻰﺿﺭﻣﻟﺍ ﺩﻧﻋ:ﻲﺋﻳﺭﻣﻟﺍ ﻉﺎﺟﺗﺭﻻﺍﻭ ﺔﻳﺩﻌﻣﻟﺍ ﺕﺎﺣﺭﻘﺗﻟﺍ, ﺔﻳﺟﻔﻌﻟﺍ ﺕﺎﺣﺭﻘﺗﻟﺍ
ﻥﻛﻣﻣﻟﺍ ﻥﻣ ﻱﻭﻣﻔﻟﺍ ﻕﻳﺭﻁﻟﺎﺑ ءﺍﻭﺩﻟﺍ ﺍﻭﺫﺧﺎﻳ ﻥﺍ ﻥﻭﻌﻳﻁﺗﺳﻳ ﻻ ﻥﻳﺫﻟﺍ
ﻰﻟﺍ ﺅﺟﻠﻟﺍ ﻝﺑﻗ ﺓﺩﺎﺗﻌﻣﻟﺍ ﺔﻳﺟﻼﻌﻟﺍ ﺓﺭﺗﻔﻟﺍ.ﻱﺩﻳﺭﻭﻟﺍ ﻥﻘﺣﻟﺎﺑ ﻎﻣ٤۰ ﻡﻬﺋﺎﻁﻋﺍ
.ﻡﺎﻳﺍ(۳-۲) ﻲﻫ ﻱﻭﻣﻔﻟﺍ ﺝﻼﻌﻟﺍ
ﻝﻛ ﺏﺳﺣ ﺔﻋﺭﺟﻟﺍ ﻝﻳﺩﻌﺗ ﻱﺭﻭﺭﺿﻟﺍ ﻥﻣ :ﻥﻭﺳﻳﻟﺍ ﺭﺟﻧﻳﻟﻭﺯ ﺔﻣﺯﻼﺗﻣ ﻲﻓ
ﺕﺎﻋﺭﺟ ﻭﺍ/ﻭ ﺔﻳﻟﺎﻋ ﺕﺎﻋﺭﺟ ﺔﻟﺎﺣﻟﺍ ﻲﻋﺩﺗﺳﺗ ﻥﺍ ﻥﻛﻣﻣﻟﺍ ﻥﻣ.ﺽﻳﺭﻣ
.ﺓﺭﺭﻛﺗﻣ ﺔﻳﻣﻭﻳ
ﻰﻁﺧﺗﺗ ﻻ ﺓﺭﺗﻔﻟ ﺏﻛﺳﻟﺍ ﻕﻳﺭﻁ ﻥﻋ ﻱﺩﻳﺭﻭﻟﺍ ﺝﻼﻌﻟﺍ ﻰﻁﻌﻳ
.ﺓﺩﺎﻋﻻﺍ ﻝﺑﻗ ﺍﺭﻭﻓ ﺏﺻﻟﺎﺑ ﺍﺩﺑﺍ.ﺔﻘﻳﻗﺩ(۳۰-۲۰)
ﻰﻠﻜﻟﺍ ﻒﺋﺎﻇﻭ ﻝﻼﺘﺧﺍ
.ءﻻﺅﻫ ﺩﻧﻋ ﺔﻋﺭﺟﻟﺍ ﻝﻳﺩﻌﺗ ﻱﺭﻭﺭﺿﻟﺍ ﻥﻣ ﺱﻳﻟ
ﺪﺒﻜﻟﺍ ﻒﺋﺎﻇﻭ ﻝﻼﺘﺧﺍ
.ءﻻﺅﻫ ﺩﻧﻋ ﺭﻳﺑﻛ ﻝﻛﺷﺑ ﺔﻳﻔﺻﺗﻟﺍ ﻝﻘﺗ
ﻦﺴﻟﺍ ﻲﻓ ﺭﺎﺒﻜﻟﺍ
.ءﻻﺅﻫ ﺩﻧﻋ ﺔﻋﺭﺟﻟﺍ ﻝﻳﺩﻌﺗ ﻱﺭﻭﺭﺿﻟﺍ ﻥﻣ ﺱﻳﻟ
ﻝﺎﻔﻃﻻﺍ
.ﻝﺎﻔﻁﻻﺍ ﺩﻧﻋ ﺝﻼﻌﻟﺍ ﺹﺧﺗ ﺓﺩﺩﺣﻣ ﺕﺎﺳﺍﺭﺩ ﻙﺎﻧﻫ ﺱﻳﻟ
ﻥﻷ ﻙﻟﺫﻟ ﺎﻌﺑﺗ ﺔﻋﺭﺟﻟﺍ ﺭﺍﺩﻘﻣ ﺏﺎﺳﺣﻟ ﻙﻟﺫﻭ ﻥﻳﺭﻓﺭﺍﻭﻠﻟ ﻥﻳﺑﻣﻭﺭﺛﻭﺭﺑﻟﺍ
ﺝﻼﻌﻟﺍ ﺓﺭﺗﻓ ﻝﻼﺧ ًﺎﻳﺭﻭﺭﺿ ﻥﻭﻛﻳ ﺩﻗ ﺔﻳﻭﺩﻷﺍ ﻩﺫﻬﻟ ﺔﻋﺭﺟﻟﺍ ﻝﻳﺩﻌﺗ
.ﻪﻓﺎﻘﻳﺍ ﺩﻌﺑ ﻙﻟﺫﻛﻭ ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺄﺑ
ﺎﻣﻬﻟﻭﺎﻧﺗ ﺩﻧﻋ ﻝﻭﺯﺎﻧﻭﻛﺍﺭﺗﺍﻭ ﻝﻭﺯﺎﻧﻭﻛﻭﺗﻳﻛ ﻥﻣ ًﻼﻛ ﺹﺎﺻﺗﻣﺍ ﺽﻔﺧﻧﻳ ﺩﻗ
ﺱﻭﻣﻳﻟﻭﺭﻛﺎﺗﻭ ﻥﻳﺳﻛﻭﺟﻳﺩ ﻥﻣ ًﻼﻛ ﺯﻳﻛﺭﺗ ﺩﺍﺩﺯﻳ ﺩﻗ .ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ﻊﻣ ﻥﻣﺍﺯﺗﻟﺎﺑ
.ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ﻊﻣ ﺎﻣﻬﻟﻭﺎﻧﺗ ﻥﻣﺍﺯﺗ ﺩﻧﻋ ﻡﺩﻟﺍ ﻲﻓ
ﺔﺑﻠﻌﻟﺍ ﻯﻭﺗﺣﻣ
ّﻱﺭﻠﻟ ﻭﺃ ﺩﻳﺭﻭﻟﺍ ﻲﻓ ﻥﻘﺣﻠﻟ ﻝﻭﻠﺣﻣﻟ ﺩﻳﻣﺟﺗﻟﺎﺑ ﻑﻔﺟﻣ ﻡﻘﻌﻣ ﻕﻭﺣﺳﻣ ﻝﺍﺯﺎﺳﻟﺃ
٤۰ ﺔﻌﺳ ﺕﺍﻭﺑﻋ ۱۰ ﻭﺍ ﺓﺩﺣﺍﻭ ﺓﻭﺑﻋ ﻰﻠﻋ ﻱﻭﺗﺣﺗ ﺔﺑﻠﻋ ﻲﻓ ﺭﻓﻭﺗﻣ ﻱﺩﻳﺭﻭﻟﺍ
.ﻝﻭﺯﺍﺭﺑﻳﻣﻭﺃ ﻡﻐﻠﻣ
ﻥﻳﺯﺧﺗﻟﺍ ﻁﻭﺭﺷ
.ءﻭﺿﻟﺍ ﻥﻋ ًﺍﺩﻳﻌﺑ ،ﻡ°۲٥ ﻯﺩﻌﺗﺗ ﻻ ﺓﺭﺍﺭﺣ ﺔﺟﺭﺩ ﻲﻓ ﻅﻔﺣﻳ
: ﻕﻳﻭﺳﺗﻟﺍ ﺔﺻﺧﺭ ﻝﻣﺎﺣ ﻭ ﻊّﻧﺻﻣﻟﺍ
ﻥﺎﻧﺑﻟ ،ﺍﺭﺩﺟ ،ﺔﻳﺋﺍﻭﺩﻟﺍ ﺕﺎﻋﺎﻧﺻﻠﻟ ﻥﺍﻭﺭﺃ
1
/
2
100%