Interaction atomes
publicité
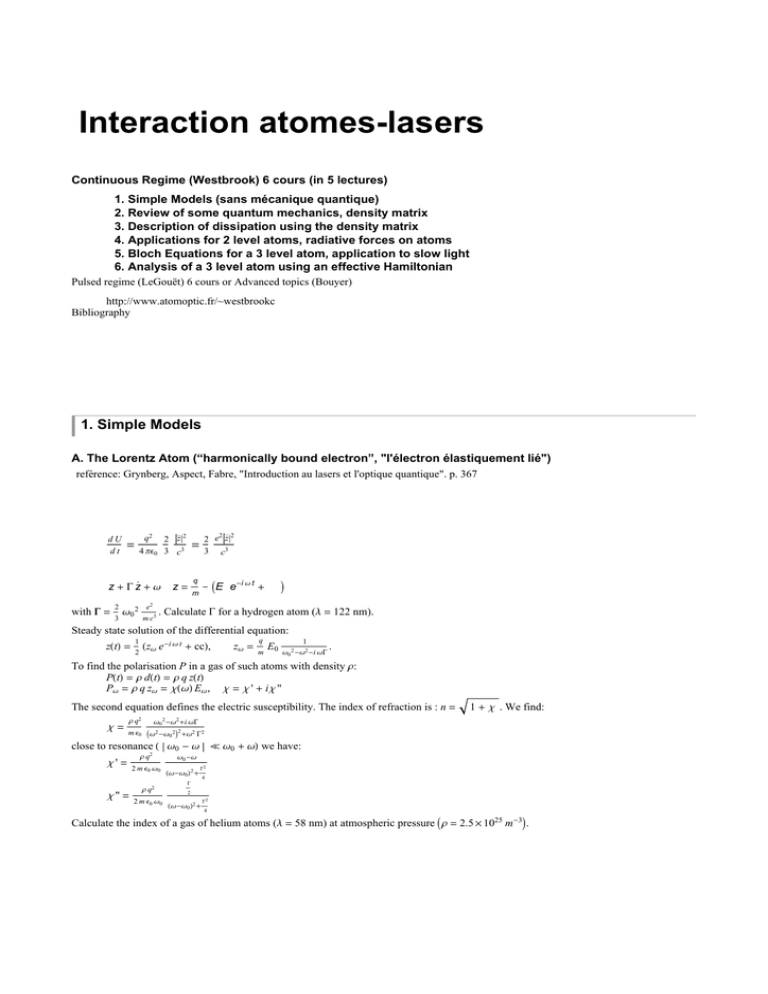
Interaction atomes-lasers Continuous Regime (Westbrook) 6 cours (in 5 lectures) 1. Simple Models (sans mé canique quantique) 2. Review of some quantum mechanics, density matrix 3. Description of dissipation using the density matrix 4. Applications for 2 level atoms, radiative forces on atoms 5. Bloch Equations for a 3 level atom, application to slow light 6. Analysis of a 3 level atom using an effective Hamiltonian Pulsed regime (LeGouët) 6 cours or Advanced topics (Bouyer) http://www.atomoptic.fr/~westbrookc Bibliography 1. C. Cohen-Tannoudji, B. Diu, F. Laloe, "Mé canique Quantique" 2. G. Grynberg, A. Aspect, C. Fabre, "Introduction aux lasers et à l'optique quantique" 3. E. Rosencher, B. Vinter, "Optoé lectronique" 4. R. Loudon "The quantum theory of light" 3rd Ed. 5. B. Cagnac, J.-P. Faroux "Lasers : Interaction lumiè re-atomes" 6. C. Cohen-Tannoudji, Jacques Dupont-Roc, G. Grynberg, "Processus d'interaction entre atomes et photons" 1. Simple Models A. The Lorentz Atom (“harmonically bound electron”, "l'é lectron é lastiquement lié ") refé rence: Grynberg, Aspect, Fabre, "Introduction au lasers et l'optique quantique". p. 367 Begin with a very simple, classical model in which we imagine an electron attached to a fixed point (the nucleus) by a spring. We approximate the electron + nucleus as a dipole. When the electron oscillates, the dipole radiates energy according to the Larmor formula (valid if the wavelength of the radiation is much larger than the electron - nucleus separation): dU dt .. .. q2 = 4 ΠΕ0 2 z2 3 c3 2 2 2 e z 3 c3 = This radiation results in a damping of the electron’ s motion, giving a differential equation for the electron position: ¨ q 1 z + G z + Ω0 2 z = IE0 e-i Ω t + ccM m 2 with G = e2 Ω0 2 3 . 3 mc 2 Calculate G for a hydrogen atom (Λ = 122 nm). Steady state solution of the differential equation: zHtL = 1 HzΩ e-i Ω t + ccL, 2 zΩ = q m E0 1 Ω0 2 -Ω2 -i ΩG . To find the polarisation P in a gas of such atoms with density Ρ: PHtL = Ρ dHtL = Ρ q zHtL PΩ = Ρ q zΩ = ΧHΩL EΩ , Χ = Χ ' + i Χ '' The second equation defines the electric susceptibility. The index of refraction is : n = Χ= Ρ q2 1 + Χ . We find: Ω0 2 -Ω2 +i ΩG m Ε0 IΩ2 -Ω0 2 M2 +Ω2 G2 close to resonance H Ω0 - Ω ` Ω0 + ΩL we have: Χ' = Χ '' = Ρ q2 2 m Ε0 Ω0 Ρ q2 2 m Ε0 Ω0 Ω0 -Ω HΩ-Ω0 L2 + G2 4 G 2 HΩ-Ω0 L2 + G2 4 Calculate the index of a gas of helium atoms (Λ = 58 nm) at atmospheric pressure IΡ = 2.5 ´ 1025 m-3 M. 2 12LM-1.nb 12 -D In[1]:= PlotB: , 2 D +14 >, 8D, - 5, 5<F 2 D +14 2.0 1.5 1.0 Out[1]= 0.5 -4 2 -2 4 -0.5 -1.0 Here G = 1 et D = Ω - Ω0 . B. Einstein’ s model of the atom ref. Loudon, The quantum theory of light, ch. 1, Kleppner, Physics Today, Feb 2005, p. 30 Two level atoms f, e, in a bath of thermal radiation with spectral and volume density u(Ω). Einstein supposed the existence of: absorption with rate: B f ®e uHΩL stimulated emission with rate: Be® f uHΩL spontaneous emission with rate: A In other words we have "Rate equations" for the populations: Π f = I-B f ®e uHΩL Π f + Be® f uHΩL Πe M + A Πe Πe = -Π f . Using the Boltzmann law: uHΩL = ÑΩ3 1 Π2 c3 eÑΩk T -1 Πe Πf = e-ÑΩ0 k T , and setting Be® f =B f ®e = B, A B = ÑΩ0 3 Π2 c3 , one can recover Planck’s formula: . Two interpretations: If you accept Planck Þ stimulated and spontaneous emission must exist. If you accept Einstein’s hypotheses Þ a demonstration of the Planck radiation law. Applications d'Einstein 1. Saturation These ideas can also be used out of equilibrium. Let u = u0 in a narrow range of frequencies DΩ around Ω0 . The steady state solution for the populations is: HB u0 + AL Πe = B u0 Π f Þ Πe = B u0 2 B u0 + A Atom cease to absorb if u0 is large. 2. Absorption, gain Suppose u = uHzL, with the same spectrum as in 1. What is the transmitted light intensity through a vapor cell of density Ρ? For u small, one finds an attenuation coefficient trouve une attenuation exponentielle avec coefficient d'absorption Ρ possibles. Comparison of the models: 1. Lorentz Input: Ω0 , q, m Basic physical quantities: d = q z, E dP Output: G, , Χ, lineshape ... dW 2. Einstein Input: Ω0 , A, B Basic physical quantities: Π f , Πe , uHΩL Output: Planck’s formula, absorption, gain, saturation ... Can we put these together? Where is the Schroedinger equation? BÑΩ c DΩ . Mais autres situations sont Basic physical quantities: d = q z, E dP Output: G, , Χ, lineshape ... dW 2. Einstein Input: Ω0 , A, B Basic physical quantities: Π f , Πe , uHΩL Output: Planck’s formula, absorption, gain, saturation ... Can we put these together? Where is the Schroedinger equation? 12LM-1.nb 3