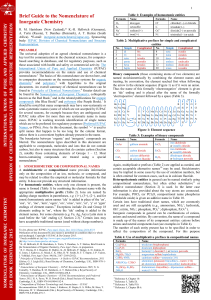
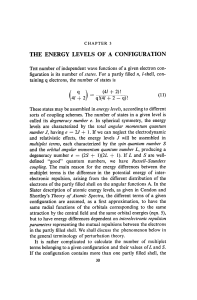
Chemical Bonding: Lewis Structures & Molecular Geometry
Telechargé par
Harani Thillainathan

125
UNIT IV: CHEMICAL BONDING
Lewis Structures (Diagrams) of covalent compounds and polyatomic ions show the bonding
arrangement of those compounds or ions.
(Chang 13th edition, section 9.6 and 9.9 read pages 381-384 and 389-395)
Practice Problems from the text: #9.43-9.47 pages 402-403
Lewis structures are based on the idea of electron sharing between atoms in order to
complete an electron octet. They show a two-dimensional bonding picture of a molecule or an
ion.
The valence electrons are represented as follows:
non-bonded electron pairs:
(free or lone pairs)
bonded electron pair(s): – (the dash represents two bonded electrons)
(shared between two atoms)
LEWIS STRUCTURES OF COVALENT COMPOUNDS AND POLYATOMIC IONS
1. A probable arrangement of the atoms can be deduced as follows:
(note: this is for neutral molecules)
The structure assumed must be consistent with the known valences of the elements:
e.g. H forms one bond,
F forms one bond,
O forms two bonds,
N forms three bonds,
C forms four bonds, etc. (
In a molecule with the formula AXn the atom A is usually the central atom to which X
are attached:
e.g. CH4, BeH2, PCl5, SF6, etc.
Atom A is also normally the least electronegative atom or the atom with the highest
valence.
ex. H2O, NH3, BF3, etc.

126
1. Draw a diagram of the molecule or polyatomic ion, showing the atoms connected by single
bonds to the central atom
2.
Add the number of valence electrons of each atom in the molecule to find the total
number of valence electrons.
Subtract the number of electrons needed to form the single bonds from the total
number of electrons.
Use the remainder to complete octets around each atom except hydrogen.
Exceptions to the octet rule: compounds of Be and B have less than 8 electrons in their
valence shell. Be ends up with 4 electrons and B has 6 electrons.
The rule is that if there are insufficient electrons to complete all the octets, complete
those of the more electronegative atoms first.
1) Incomplete octet: BeCl2, BF3, SnF2, and HgCl2
2) Molecules/ions with an odd number of electrons: NO, NO2
3) Expanded octet: PCl5, SF6, XeF4 etc… (Noble gases only have expanded octets)
Examples:
CH4
H2O
NH3

127
Exceptions to the octet rule (Incomplete octet):
BeCl2
BeH2
BF3
BH3

128
3. If the molecule is charged, and/or a polyatomic ion, add one electron for each negative charge
or subtract one electron for each positive charge.
Examples:
H3O+
NH4+
BCl4–
NH2–

129
4. Atoms from the 3rd or later period:
If the central atom is from period 3 or a later period, the octet rule may not apply. These
atoms can have more than 8 electrons in their valence shell: (Expanded octet)
Examples:
PCl5 SF6
If there are more than enough electrons to complete all octets, add the remaining
electrons in pairs to the central atom (which must be from the 3rd or a later period).
Examples:
SCl4 ClF3
XeF2 BrF4+
SbCl52–
 6
6
 7
7
 8
8
 9
9
 10
10
 11
11
 12
12
 13
13
 14
14
 15
15
 16
16
 17
17
 18
18
 19
19
 20
20
 21
21
 22
22
 23
23
 24
24
 25
25
 26
26
 27
27
 28
28
 29
29
 30
30
 31
31
 32
32
 33
33
1
/
33
100%