Analyse Dimensionnelle : Cours de Génie des Procédés
Telechargé par
BOUSSOUF Ibtissam

LST GP Analyse Dimensionnelle
- 1 -
A
AN
NA
AL
LY
YS
SE
ED
DI
IM
ME
EN
NS
SI
IO
ON
NN
NE
EL
LL
LE
E
La base de l’analyse dimensionnelle est la théorie de la similitude qui rassemble en même
temps le côté théorique et pratique du phénomène à étudier. Elle réduit le nombre de
variables indépendant qui décrivent l’opération physique.
La procédure principale de l’analyse dimensionnelle peut être résumée comme suite :
1. Compiler une liste de variables pertinentes soit dépendante ou indépendante pour le
problème considéré,
2. Utiliser une technique appropriée pour identifier le nombre et la forme des
nobmres sans dimension.
Une de ces techniques utilisée est le théorème Pi de Buckingham. Elle traite les variables et
les présentes sous une forme pratique indépendantes des unites et échelles.
L’analyse dimensionnelle est un puissant outil en genie des procédés.
1 Dimension et unités
1.1 Les systèmes d'unités
Une unité est une grandeur prise comme terme de comparaison avec des grandeurs de la
même espèce. Les nombres qui résultent de ces comparaisons en donnent les mesures.
l.l.l Le système c.g.s.
Dans ce système nous avons, pour les grandeurs que l'on mesure généralement, les 4 unités
suivantes :
Longueur centimètre cm
Masse gramme g
Temps second s
Force dyne d
1.1.2 Le système international SI

LST GP Analyse Dimensionnelle
- 2 -
Longueur mètre m
Masse kilogramme kg
Temps second s
Force Newton N = kg . m . s2
Température degré centigrade °C
1.1.3 Le système anglais
Longueur foot ft
Masse pound 1 bm
Temps seconde s
Force poundal 1 bF
Température degré Farenheit °F
1.1.4 Le système américain
Longueur foot ft
Masse pound mass lbm
Temps seconde s
Force pound force lbf
Température degré Farenheit °F
1.2 Les dimensions
Si les unités varient, toute grandeur n'a qu'une seule dimension.
Reprenons les grandeurs de définition des systèmes d'unités avec leur dimension :
Longueur L(indépendante)
Temps t(indépendante)
Masse M(indépendante)
Force F(dépendante)
Température T(indépendante)

LST GP Analyse Dimensionnelle
- 3 -
Grandeurs physique
Symbole
dimension
Unité, SI
Unités fondamentales
Longueur
Temps
Masse
Température
l
t
m
T
L
T
M
θ
m
s
Kg
°K degré Kelvin
En choisissant L, T, et M comme dimensions indépendantes, nous pouvons retrouver la force,
le travail, l’énergie cinétique, la pression …
Unités dérivées
Vitesse
Accélération
Force
Débit
Pression
Contrainte
Travail
Energie
Quantité de chaleur
Viscosité dynamique
Viscosité cinématique
Tension superficielle
ϑ
a
F
Ḋ
P
τ
W
E
ΔQ
η
γ
σs
[ϑ] = L.T-1
[a] = M.L.T-2
[F] = L.T-2
[Ḋ] = L3. T-1
[P] = M . L-1 . T-2
[τ] = M . L-1 . T-2
[W] = M . L2. T-2
[E] = M . L2. T-2
[ΔQ] = [E]
[η] = M . L-1 . T-1
[γ] = L2. T-1
[σs] = M . T-2
m.s-1
m.s-2
Kg.m.s-2 =N
m3.s-1
kg.m-1.s-2 =N.m-2 =Pa
Pa
N.m = J = le joule
Kg.m2.s-2 =J
J
Kg.m-1.s-1
m2.s-1
N.m-1 = Kg.s-2
Rq : Toute énergie est de dimension M . L2. t-2.
1.3 Les températures absolues
La température n'est pas une énergie : on peut la considérée comme une échelle de désordre !
En effet l'ordre parfait, l'immobilité totale, l'absence de tout mouvement va nous définir le
zéro absolu. En revanche, côté désordre, l'échelle reste ouverte.
L'échelle des températures °C ou °F est complètement arbitraire.
La relation qui permet de passer de °F en °C et inversement est la suivante
T (°C) = 5/9 T(°F) - 32

LST GP Analyse Dimensionnelle
- 4 -
II existe toutefois une échelle de température dont le zéro est la température à laquelle il
n'existe plus de mouvement moléculaire d'un gaz parfait. Il y a deux types d'unités pour cette
échelle
Le Kelvin, K, qui est l'unité S1
T (K) = T (°C) + 273,15
Le Rankine, R
T (R) = T (°F) + 459,58
Le Kelvin et le Rankine ne doivent pas être considérés comme des degrés mais bien comme
des unités.
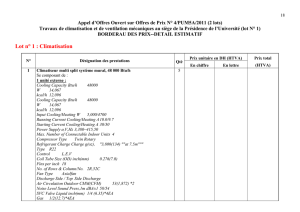
Tableau 1 - Grandeurs, unités et dimensions. Conversions des principales unités.
LES CONSTANTES ET LEURS DIMENSIONS
g =32,1739 ft lb/lbfs2
= 4,1697.108 ft lb/lbfh2
= 1 g cm/ds2
= 1 kg m/N s2
= I Ib ft/poundal s2
R constante des gaz parfaits
= 1544 ft lbf/Ib mol R
=0,730 ft3atm/Ib mol R
=0,08205 m3atm/kg mol K
= 8,314 J/g mol K
= 1,987 cal/g mol K
σconstante de Stefan-Boltzmann
= 0,1714.10-8 Btu/h ft2R4
= 5,6697.108 W/m2K4
CHALEUR SPÉCIFIQUE
1 Btu /lb °F = I kcal/kg °C = l cal/g °C
1 Btu/lb °F = 4186,69 J/kg °C
1 Btu/1b °F = 4, I 8669 J/g K
1 J/g °C = 0,23885 Btu/lb °F
COEFFICIENT DE TRANSFERT
DE CHALEUR
1 Btu/h ft2°F = 5,677 W/m2°C
I Btu/h ft2°F = 5,677.10-4 W/ cm2°C
1 W/ m2°C = 0,1761 Btu/h ft2°F
1 Btu/h ft2°F = 4,882 kcal/h m2°C
CONDUCTIVITÉ THERMIQUE
I Btu/h ft °F = 1,7303 W/m °C
1 Btu/h ft °F = 1,7303.10-2 W/cm °C
I Btu/h ft °F = 0,4132 cal/s m °C
1 W/m°C = 0,5779 Btu/h ft °F
1 W/cm°C = 57,79 Btu/h ft °F
DEBIT THERMIQUE
1 Btu/h ft3- 10,35 W/m3
I Btu/h ft3=- 8,9 kcal/h m3
1 W/ m3= 0,0966 Btu/h ft3
DIFFUSIVITE
1 ft2/s = 0,0929 m2/s
1 ft2/h = 0,2581 cm2/s
I ft2/h = 0,2581.10-4 m2/s
1 m2/s = 10,7639 ft2/s
ÉNERGIE, CHALEUR, PUISSANCE
1J= 1 W s= 1 Nm
1 Btu = 1055,04 J
1 Btu = 1055,04 W s
1 Btu = 1055,04 N m
1 Btu = 252 cal
1 Btu = 0,252 kcal
I Btu = 778, I 61 ft lbf
1 Btu/h =0,2931 W
1 Btu/h=0,2931.10-3 kW
1 Btu/h = 3,93.10-4 CV
1 cal = 4,1868 J (ou W s ou N m)
1 cal = 3,968.10-3Btu
1 kcal = 3,968 Btu
1 C V = 550 ft lbf/s
1 CV = 745,7 W
1Wh = 3,413 Btu
1 kWh = 3413 Btu
MASSE
1 lb = 453,6 g
1 lb = 0,4536 kg
1 kg =2,2046 Ib
LONG UEUR
1 Á = 10-10 m
1 μm= 10-3 mm
1 μm = 10-6 m
I in = 2,54 cm
1 in = 2,54 10-2 m
1 ft = 0,3048 m
1 m = 3,2808 ft
1 mile = 1609,34 m
1 mile = 5280 ft
MASSE VOLUMIQUE
I lb/ìn3= 27,680.103kg/m3
1 lb/ft3= 16,019 kg/ m3
1 kg/ m3= 0,06243 lb/ ft3
1 lb mol/ ft3= 16,019 kg mol/ m3
1 kg mol/ m3= 0,06243 lb mol/ ft3
PRESSION, FORCE
1 N = 1 kg m/ s2
1 N = 0,22481 Ibf

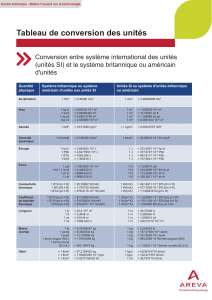
LST GP Analyse Dimensionnelle
- 5 -
l cm2/s = 3,8745 ft2/h
FLUX MASSIQUE
1 Ib mol/ft2h =1,3563.10-3 kg mol/m2s
1 kg mol/ m2s = 737,3 Ib mol/ft2h
1 Ib/ ft2h = 1,3563. 10-3 kg/ m2s
1 lb/ ft2s = 4,882 kg/ m2s
1 kg / m2s = 737,3 Ib/ ft2h
1 kg/ m2s = 0,2048 Ib/ ft2s
FLUX THERMIQUE
1 Btu/h ft2= 3,1537 W/ m2
I Btu/h ft2= 3,1537.10-3 kW/ m2
1 W/ m2=0,31709 Btu/h ft2
Verso…
SURFACE
1 in2= 6,4516 cm2
I in2= 6,4516. 10-4m2
1 ft2= 929 cm2
1 ft2= 0,0929 m2
I m2= 10,764 ft2
TEMPÉRATURE
I K=1,8 R
T (°F) = 1,8(K - 273) + 32
T (K) = 1/1,8.(R - 492)
ΔT (°C) = 1,8 ΔT(°F)
TENSION SUPERFICIELLE
1 Ibf/ft = 14,5937 N/m
1 N/m = 0,0685291 Ibf/ft
VISCOSITÉ
I poise = 1 g/cm s
I poise = 102centipoises
I poise = 241,9 Ib/ft h
1 N = 105dyne
1 lbt = 32,174 ft Ib/ s2
1 Ibf = 4,4482 N
1 Ibf= 4,4482 kg m/ s2
1 Ibf/ in2= 1 psi = 6894,76 N/ m2
1 Ibf/ ft2= 47,880 N/ m2
1 N/ m2= 1 Pa
1 bar = 105;N/ m2= 105Pa
1 atm = 14,696 Ibf/in2
1 atm = 21 16,2 Ibf/ft2
1 atm = 1,0132 105N/m2
1 atm= 1,0132 bar
1 centipoise = 2-419 Ib/ft h = 10-3 Pa s
1 Ib/ft s = 1,4882 kg/m s
1 Ib/ft s = 14,882 poises
1 Ib/ft s = 1488,2 centipoises
1 lb/ft h = 0,4134 10-3 kg/m s
1 Ib/ft h = 0,4134. 10-2 poise
1 lb/ft h = 0,4134 centipoise
VITESSE
1 ft/s = 0,3048 m/s
1 m/s = 3,2808 ft/s
1 mile/h = 1,4667 ft/s
1 mile/h = 0,44704 m/s
VOLUME
1 in3= 16,387 cm3
1 cm3= 0,06102 in3
1 ft3= 28,3 I 68 l
1 ft3= 7,4805 gal (U.S.)
I m3= 35,315 ft3
1 gal (U.S.) = 3,7854 10-3 m3
1 gal (U.S.) = 0,13368 ft3
Procédure à suivre dans un problème d’analyse dimensionnelle :
Identifier toutes les variables indépendantes intervenant dans le problème étudié, soit
au nombre N,
Spécifier les dimensions de ces variables en utilisant les dimensions de bases
(L, T, M, θ),
Choisir les grandeurs convenables, disons au nombre D,
Utiliser une méthode appropriée pour identifier le nombre et la forme des paramètres
sans dimensions.
 6
6
 7
7
 8
8
 9
9
 10
10
 11
11
 12
12
1
/
12
100%