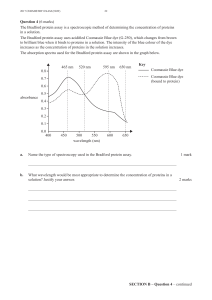
CSC Poster 2006

How to quantify gases in air with an open-path FTIR
or a variable pathlength cell
Denis Bussières, Dépt. Sc. Fondamentales, Université du Québec à Chicoutimi, Saguenay, QC
Since the middle of the 90’s several FTIR instruments are available to measure and
quantify gases in the open atmosphere. A typical setup (monostatic) looks like this :
FTIR
Mirror
Telescope IR
beam
Before going outside, it is easier to get spectra in the lab and make sure of the results.
So, spectra were taken with a Bruker IFS66 FTIR at 0,5 cm-1 resolution (aperture of 2,5
mm) and a Wilks « variable path » cell (0,75-21,75 m) equipped with BaF2 windows.
The way the spectra were taken, the reference spectrum was made with the cell empty
(pressure < 1 Torr) then the gas inserted into the cell and completed to atmospheric
pressure with N2.
By doing so, a series of spectra were taken by varying the optical pathlength in the cell
(from 0,75 to 11,25 m). These spectra showed a moving baseline due to pathlength
difference between the gas spectrum and the reference spectrum (see Figure 1).
A usual feature in open atmosphere spectra is the presence of bands in the 1400-1800,
2250 and 3500-4000 cm-1 regions (see Figure 6). These bands are due to the ubiquitous
presence of H2O and CO2 which were avoided in the lab setup.
The baseline was flattened close to zero absorbance by taking reference points on each
side of the region of importance and assuming a linear variation with the wavenumbers.
As a simple verification, the mean baseline absorbance value between 1260 and 1270
cm-1 had a value lower than 1 milliabsorbance (see Figure 4).
One peak of SO2, at 1373.8 cm-1, is shown in Figure 5 to increase linearly with optical
pathlength as expected.
From the good old Beer-Lambert’s law(2) : Abs = ε n l
Where ε is in units of cm2 molec-1
n in units of molec cm-3
l in units of cm
The sum of the line intensities over a whole band has to be done to get a comparable
value(3) :
Σ ε x FWHM (cm-1) → Σ S (cm molec-1)
Here the FWHM was used instead of the Doppler halfwidth for a matter of availability
(medium resolution spectra).
Then the line intensities (S) is related to the Einstein A coefficient (4) :
Σ S = A gu A
gl 8 ¶ nr3 c ν2
where A : Einstein A coefficient (spontaneous emission)
gu and gl : degeneracies of upper and lower states (assumed to be ~1)
nr : refraction index (taken as 1.00027) c : speed of light (2.9979x1010 cm s-1)
ν : mean wavenumber of the transition (950, 1362 and 1617 cm-1 respectively)
NH3 spectra (~1300 ppmv)
-0.5
0
0.5
1
1.5
2
700 800 900 1000 1100 1200
Wavenumbers (cm-1)
Absorbance
0.75m
6.75m
SO2 spectrum corrected (at 9.75m et ~1.18 ppm)
-0.02
0.00
0.02
0.04
0.06
0.08
0.10
1250 1270 1290 1310 1330 1350 1370 1390
Wavenumbers (cm-1)
Absorbance (baseline corrected)
SO
2
spectra (~1.18ppmv)
-0.2
0
0.2
0.4
0.6
0.8
1
1230 1330 1430 1530 1630 1730 1830 1930 2030
Wavenumbers (cm-1)
Absorbance
0.75m
3.75m
5.25m
NO
2
spectra (22.5ppmv)
-0.1
0.1
0.3
0.5
0.7
0.9
1000 1500 2000 2500 3000
Wavenumbers (cm-1)
Absorbance
0.75m
3.75m
6.75m
9.75m
Σ S (cm molec-1)
Difference
Hitran database This work
NH3 (630-1244 cm-1) 8.78 x 10-20 6.49 x 10-20 * -26 %
SO2 (1311-1400 cm-1) 3.08 x 10-17 2.71 x 10-17 -12 %
NO2 (1429-1837 cm-1) 5.69 x 10-17 4.72 x 10-17 -17 %
* From 700 cm-1 only
Sincere thanks to Dr. G. Harris and his team members at the Center for Atmospheric Chemistry, York University for
receiving me in his lab and helping me for this work.
Thanks to the Université du Québec à Chicoutimi for supporting me financially through this work.
(1) Shao, L., Griffiths, P.R., Chu, P.M. and Vetter, T.W., Appl. Spectro., 60, 3, pp.254-260 (2006).
(2) Pouchet, I., Zéninari., V., Parvitte, B. and Durry, G., J. Quant. Spect. & Rad. Transf., 83, pp.6119-628 (2004).
(3) Atkins, P. and de Paula, J., Physical Chemistry, 7th edition, 2002, W.H.Freeman and Co., New York, 1140 pages.
(4) Newman, S.M., Lane, I.C., Orr-Ewing, A.J., Newnham, D.A. and Ballard, J., J.Chem.Phys., 110, 22, pp.10749-10757 (1999).
Einstein A coefficient (s-1)
Difference
Hitran database This work
NH3 33 0.044 - 99.9 %
SO2 47 38 - 9 %
NO2 161 93 - 30 %
Figure 1. Figure 2.
Figure 4.
Figure 3.
When not respecting the Beer-Lambert law(1) (see Figures 1 and 2), spectra may be
distorted and care was taken to avoid it.
Table 1.
Table 2.
Respecting the Beer-Lambert law allows the quantification of gases in agreement with
accepted values (HITRAN). Ammonia result is three orders of magnitude off the reference
value because of a distorted spectrum by too high absorbance.
Warm car exhaust diluted 621 and 3122 times in N2 (at 2,25 m)
0.1
0.3
0.5
0.7
0.9
1.1
700 1200 1700 2200 2700 3200 3700
Wavenumbers (cm
-1
)
Absorbance
320 ppmv
1610 ppmv
CO2
mainly H2O
H2O
CO2
CH
4
C
2
H
2
and C
2
H
4
all lost in the grass
CO
very tiny
Figure 6.
A warm car exhaust sample (Volvo 2000) was
aspirated into the cell (through 5µ filter) and
diluted with N2 to give spectra in Figure 6.
Spectra of H2O and CO2 with the exact same
treatment would be needed to subtract them
from the ones on Figure 6 to be able to get
the trace contaminant in the exhaust.
Figure 5.
Absorbance at 1373.8 cm-1 vs. pathlength (1.18 ppmv SO2)
y = 0.0031x + 0.0517
R
2
= 0.9648
0.05
0.06
0.07
0.08
0.09
0 2 4 6 8 10 12
Pathlength (m)
Absorbance (baseline corrected
1
/
1
100%