Dietary intake and biomarkers of acrylamide exposure and risk

Dietary intake and biomarkers
of acrylamide exposure and risk
of endometrial and ovarian cancer
A molecular epidemiologic study in the European
Prospective Investigation into Cancer and Nutrition
Mireia Obón Santacana
Aquesta tesi doctoral està subjecta a la llicència Reconeixement- NoComercial –
SenseObraDerivada 3.0. Espanya de Creative Commons.
Esta tesis doctoral está sujeta a la licencia Reconocimiento - NoComercial – SinObraDerivada
3.0. España de Creative Commons.
This doctoral thesis is licensed under the Creative Commons Attribution-NonCommercial-
NoDerivs 3.0. Spain License.

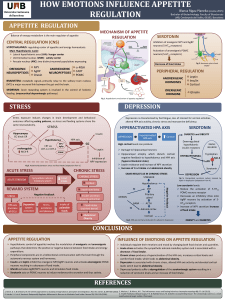
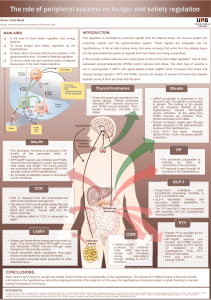
RESULTS


RESULTS
57
5. RESULTS
The present chapter introduces the five publications presented in this thesis, which have been
published in international journals. Each publication is preceded by a brief summary in Catalan.
Table 10. Impact factor, category, and journal rank of the articles presented in this thesis
Articles Reference1
IF2
Category & Journal rank2
Obón-Santacana, M. et al. Dietary intake of acrylamide and
endometrial cancer risk in the European Prospective Investigation into
Cancer and Nutrition cohort. Br. J. Cancer 111, 987–997 (2014).
4.836 Oncology - Quartile 1
Obón-Santacana, M. et al. Dietary intake of acrylamide and epithelial
ovarian cancer risk in the european prospective investigation into
cancer and nutrition (EPIC) cohort. Cancer Epidemiol. Biomark. Prev.
Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 24,
291–297 (2015).
4.125
Public, environmental &
occupational health -
Quartile 1
Obón-Santacana, M. et al. Dietary and lifestyle determinants of
acrylamide and glycidamide hemoglobin adducts in non-smoking
postmenopausal women from the EPIC cohort. Eur. J. Nutr. (2016).
doi:10.1007/s00394-016-1165-5
3.467
Nutrition & dietetics –
Quartile 1
Obón-Santacana, M. et al. Acrylamide and glycidamide hemoglobin
adduct levels and endometrial cancer risk: A nested case-control study
in nonsmoking postmenopausal women from the EPIC cohort. Int. J.
Cancer 138, 1129–1138 (2016).
5.085 Oncology - Quartile 1
Obón-Santacana, M. et al. Acrylamide and Glycidamide Hemoglobin
Adducts and Epithelial Ovarian Cancer: A Nested Case-Control Study in
Nonsmoking Postmenopausal Women from the EPIC Cohort. Cancer
Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored
Am. Soc. Prev. Oncol. 25, 127–134 (2016).
4.125
Public, environmental &
occupational health -
Quartile 1
IF; Impact Factor
1. Ordered by thesis presentation
2. Accessed date: 10 June 2016 (Journal Citation Reports ® of the ISI Web of Knowledge
SM
, Thompson Reuters)

 6
6
 7
7
 8
8
 9
9
 10
10
 11
11
 12
12
 13
13
 14
14
 15
15
 16
16
 17
17
 18
18
 19
19
 20
20
 21
21
 22
22
 23
23
 24
24
 25
25
 26
26
 27
27
 28
28
 29
29
 30
30
 31
31
 32
32
 33
33
 34
34
 35
35
 36
36
 37
37
 38
38
 39
39
 40
40
 41
41
 42
42
 43
43
 44
44
 45
45
 46
46
 47
47
 48
48
 49
49
 50
50
 51
51
 52
52
 53
53
 54
54
 55
55
 56
56
 57
57
 58
58
 59
59
 60
60
 61
61
 62
62
 63
63
 64
64
 65
65
 66
66
 67
67
 68
68
 69
69
 70
70
 71
71
 72
72
 73
73
 74
74
 75
75
1
/
75
100%