Annexe D

Octobre 2005
Examen prototype de 12e année
Chimie
Code du cours : 8222
Code à barres
Mois Jour
Date de naissance
Annexe D
Pour plus d’information,
voir le Tableau des
spécifications.

- i -
(Chimie – Examen prototype)
(octobre 2005)
Chimie
Durée : Deux heures et demie
L’examen de chimie se fait à livre ouvert. Vous pouvez apporter avec vous tous les manuels autorisés
que vous voulez, ainsi que vos notes de cours. Le manuel de laboratoire peut faire partie de vos notes
de cours.
Les calculatrices peuvent être employées. Seules les calculatrices silencieuses à main conçues pour
les fonctions mathématiques telles que les opérations logarithmiques, trigonométriques et
graphiques sont autorisées. Les ordinateurs, les calculatrices à clavier QWERTY, les calculatrices
capables de manipulation symbolique et les tablettes électroniques ne sont pas autorisés. Les
calculatrices possédant des notes incorporées (définitions ou explications en notation alpha) qui ne
peuvent pas être effacées ne sont pas autorisées. Tous les programmes doivent être effacés de toutes
les calculatrices.
Vous avez droit à un dictionnaire imprimé. Aucune autre forme de dictionnaire (par exemple
électronique) ni aucun dictionnaire bilingue n’est permis.
Ne vous attardez pas trop sur l’une ou l’autre des questions. Lisez attentivement les questions.
Toutes les questions sont à choix multiple et seront corrigées à la machine. Vous devez inscrire toutes vos
réponses sur la feuille de réponses intitulée «Student Examination Form». Quatre réponses différentes
sont proposées pour chaque question, dont l’une est meilleure que les autres. Choisissez la meilleure
réponse, et notez-la sur la feuille de réponses comme dans l’exemple ci-dessous
Exemple : Réponses :
1. Dans laquelle des substances suivantes le
soufre a-t-il le degré d’oxydation le plus élevé?
A. H2S
B. H2SO4
C. SO2
D. Na2S2O3
Feuille de réponses informatisée :
1. A B C D E
Utilisez un crayon ordinaire HB pour inscrire vos réponses sur la feuille informatisée. Pour changer
une réponse, il faut d’abord effacer complètement la première. Il ne doit y avoir qu’une seule
réponse par question. Effacez aussi tous les autres traits de crayon de votre feuille de réponses. Si
vous avez besoin de brouillon, écrivez dans l’espace qui se trouve à côté de chaque question du cahier
d’examen.
Ne pliez ni la feuille de réponses, ni le cahier d’examen. N’oubliez pas de remplir le cadre bleu
d’identification de votre feuille de réponses.
Quand l’examen est terminé, placez la feuille de réponses sous le cahier d’examen, et insérez le tout
dans la même enveloppe. N’oubliez pas de sceller l’enveloppe, de remplir la fiche d’identification du
candidat et d’inscrire les renseignements demandés sur le dessus de l’enveloppe.

- ii -
(Chimie – Examen prototype)
(octobre 2005)
Chimie 30
Les tableaux suivants sont fournis avec cet examen :
• Solubilité dans l’eau de composés courants
• Force relative des acides en solution aqueuse à température ambiante (25 °C)
• Potentiels d’électrodes normaux des demi-réactions
• Tableau périodique des éléments
• Zone de pH des indicateurs acide-base courants
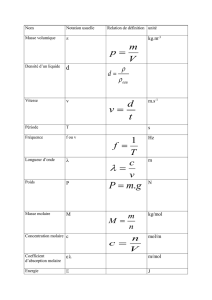
• Formulaire
Solubilité dans l’eau de composés courants
Règle Ions négatifs Ions positifs Solubilité
1 pratiquement tous +
Li , +
Na , +
K
, +
Rb , +
Cs , +
Fr soluble
2 pratiquement tous +
H soluble
3 pratiquement tous +
4
NH soluble
4 nitrate, −
3
NO pratiquement tous soluble
+
Ag solub. faible
5
acétate, −
COOCH3 tous les autres soluble
+
Ag , 2
Pb+, 2
2
Hg +, +
Cu , +
Tl solub. faible
6 bromure, −
Br
chlorure, −
Cl
iodure, −
I tous les autres soluble
2
Ca+, 2
Sr+, 2
Ba+, 2
Ra+, 2
Pb+, +
Ag , 2
2
Hg + solub. faible
7
sulfate, 2
4
SO − tous les autres soluble
+
Li , +
Na , +
K
, +
Rb , +
Cs , +
Fr , +
H, +
4
NH ,
2
Be+, 2
Mg+, 2
Ca+, 2
Sr+, 2
Ba+, 2
Ra+
soluble
8
sulfure, 2
S−
tous les autres solub. faible
+
Li , +
Na , +
K
, +
Rb , +
Cs , +
Fr , +
H, +
4
NH ,
2
Sr+, 2
Ba+, 2
Ra+, +
Tl
soluble
9
hydroxyle, −
OH
tous les autres solub. faible
+
Li , +
Na , +
K
, +
Rb , +
Cs , +
Fr , +
H, +
4
NH soluble
10 carbonate, 2
3
CO −
phosphate, 3
4
PO −
sulfite, 2
3
SO −
tous les autres solub. faible
Une substance est considérée comme soluble si elle se dissout suffisamment pour donner une concentration
ionique supérieure à 0,1 mole par litre à température ambiante.
(Adapté de
Chemistry: Experimental Foundations
, 4th Edition. Prentice-Hall, Inc., 1987.)

- iii -
(Chimie – Examen prototype)
(octobre 2005)
FORCE RELATIVE DES ACIDES EN SOLUTION AQUEUSE À TEMPÉRATURE AMBIANTE (25 °C)
Acide Réaction Ka
acide perchlorique HClO4 → H+(aq) + ClO4–(aq) très grande
acide iodhydrique HI(aq) → H+(aq) + I–(aq) 3,2 × 109
acide bromhydrique HBr(aq) → H+(aq) + Br–(aq) 1,0 × 109
acide clorhydrique HCl(aq) → H+(aq) + Cl–(aq) 1,3 × 106
acide sulfurique H2SO4(aq) → H+(aq) + HSO4–(aq) 1,0 × 103
acide nitrique HNO3(aq) → H+(aq) + NO3–(aq) 2,4 × 101
acide oxalique HOOCCOOH(aq) → H+(aq) + HOOCCOO–(aq) 5,4 × 10–2
acide sulfureux (SO2 + H2O) H2SO3(aq) → H+(aq) + HSO3–(aq) 1,7 × 10–2
ion hydrogénosulfate HSO4–(aq) → H+(aq) + SO4–2(aq) 1,3 × 10–2
acide phosphorique H3PO4(aq) → H+(aq) + H2PO4–(aq) 7,1 × 10–3
tellurure d’hydrogène H2Te(aq) → H+(aq) + HTe–(aq) 2,3 × 10–3
acide fluorhydrique HF(aq) → H+(aq) + F–(aq) 6,7 × 10–4
acide nitreux HNO2(aq) → H+(aq) + NO2–(aq) 5,1 × 10–4
séléniure d’hydrogène H2Se(aq) → H+(aq) + HSe–(aq) 1,7 × 10–4
acide benzoïque C6H5COOH(aq) → H+(aq) + C6H5COO–(aq) 6,6 × 10–5
acide acétique CH3COOH(aq) → H+(aq) + CH3COO–(aq) 1,8 × 10–5
acide carbonique (CO2 + H2O) H2CO3(aq) → H+(aq) + HCO3–(aq) 4,4 × 10–7
acide sulfhydrique H2S(aq) → H+(aq) + HS–(aq) 1,0 × 10–7
ion dihydrogénophosphate H2PO4–(aq) → H+(aq) + HPO4–2(aq) 6,3 × 10–8
ion hydrogénosulfite HSO3–(aq) → H+(aq) + SO3–2(aq) 6,2 × 10–8
acide hypochloreux HClO(aq) → H+(aq) + ClO–(aq) 2,9 × 10–8
ion ammonium NH4+(aq) → H+(aq) + NH3(aq) 5,7 × 10–10
ion hydrogénocarbonate HCO3–(aq) → H+(aq) + CO3–2(aq) 4,7 × 10–11
ion hydrogénotellurure HTe–(aq) → H+(aq) + Te–2(aq) 1,0 × 10–11
péroxyde d’hydrogène H2O2(aq) → H+(aq) + HO2–(aq) 2,4 × 10–12
ion hydrogénophosphate HPO4–2(aq) → H+(aq) + PO4–3(aq) 4,4 × 10–13
ion hydrogénosulfure HS–(aq) → H+(aq) + S–2(aq) 1,2 × 10–15
ammoniaque NH3(aq) → H+(aq) + NH2–(aq) très faible

- iv -
(Chimie – Examen prototype)
(octobre 2005)
Potentiels d’électrodes normaux des demi-réactions
Concentrations ioniques de 1,0 mol/L dans l’eau, à 25 °C. Tous les ions sont aqueux.
Demi-réaction E° (Potentiel)
(volts)
−− →+ F2e2)g(F2 +2,87
OH4Mne5H8MnO 2
2
4+→++ +−+
− +1,52
)s(Aue3Au 3→+ −+ +1,50
−− →+ Cl2e2)g(Cl2 +1,36
OH7Cr2e6H14OCr 2
3
2
72 +→++ +−+
− +1,33
OH2Mne2H4)s(MnO 2
2
2+→++ +−+ +1,28
OHe2H2)g(O 22
2
1→++ −+ +1,23
−− →+ Br2e2)(Br2l +1,06
OH2)g(NOe3H4NO 23 +→++ −+
− +0,96
)s(AgeAg →+ −+ +0,80
OH)g(NOeH2NO 223 +→++ −+
− +0,78
23 FeeFe +−+ →+ +0,77
−− →+ I2e2)s(I2 +0,53
)s(Cue2Cu 2→+ −+ +0,34
OH2)g(SOe2H4SO 22
2
4+→++ −+
− +0,17
24 Sne2Sn +−+ →+ +0,15
)g(SHe2H2)s(S 2
→++ −+ +0,14
)g(He2H2 2
→+ −+ 0,00
)s(Fee3Fe 3→+ −+ – 0,04
)s(Pbe2Pb 2→+ −+ – 0,13
)s(Sne2Sn 2→+ −+ – 0,14
)s(Nie2Ni 2→+ −+ – 0,25
)s(Cde2Cd 2→+ −+ – 0,40
)s(Fee2Fe 2→+ −+ – 0,44
)s(Cre3Cr 3→+ −+ – 0,74
)s(Zne2Zn 2→+ −+ – 0,76
)s(Mne2Mn 2→+ −+ –1,18
)s(Ale3Al 3→+ −+ –1,66
)s(Mge2Mg 2→+ −+ –2,37
)s(NaeNa →+ −+ –2,71
)s(Cae2Ca 2→+ −+ –2,87
)s(Bae2Ba 2→+ −+ –2,90
)s(CseCs →+ −+ –2,92
)s(KeK →+ −+ –2,92
)s(LieLi →+ −+ –3,00
 6
6
 7
7
 8
8
 9
9
 10
10
 11
11
 12
12
 13
13
 14
14
 15
15
 16
16
 17
17
 18
18
 19
19
 20
20
 21
21
 22
22
 23
23
 24
24
 25
25
 26
26
 27
27
 28
28
 29
29
 30
30
 31
31
 32
32
 33
33
1
/
33
100%