NUTRICASUS NUTRICASUS

Editorial
Chers Docteurs
Tout d'abord, nous vous présentons nos meilleurs vœux pour l'année 2006. Nous avons
l'honneur de vous faire parvenir le premier numéro de votre nouveau mensuel: NUTRICASUS.
Ce Journal est votre journal. Il se veut un outil de communication et de formation continue dans
le domaine de la médecine nutritionnelle et fonctionnelle. NUTRICASUS vous présentera
chaque mois un cas clinique mettant en évidence l'intérêt de la biologie nutritionnelle pour aider
efficacement vos patients atteints des affections les plus fréquentes ou désirant conserver ou
optimiser leur capital santé. Des références scientifiques validant les concepts décrits seront
incluses. NUTRICASUS tentera également de répondre aux questions les plus souvent posées
par rapport à la biologie nutritionnelle tant au niveau scientifique que logistique. Enfin
NUTRICASUS ouvre un espace pour vous permettre de vous exprimer librement par rapport à
la médecine nutritionnelle. En espérant que NUTRICASUS deviendra votre compagnon
incontournable dans votre pratique, nous vous réitérons nos souhaits de santé et de succès
pour 2006.
Nutritionnellement vôtre
Ellipsys S.A.
Editorial
Chers Docteurs
Tout d'abord, nous vous présentons nos meilleurs vœux pour l'année 2006. Nous avons
l'honneur de vous faire parvenir le premier numéro de votre nouveau mensuel: NUTRICASUS.
Ce Journal est votre journal. Il se veut un outil de communication et de formation continue dans
le domaine de la médecine nutritionnelle et fonctionnelle. NUTRICASUS vous présentera
chaque mois un cas clinique mettant en évidence l'intérêt de la biologie nutritionnelle pour aider
efficacement vos patients atteints des affections les plus fréquentes ou désirant conserver ou
optimiser leur capital santé. Des références scientifiques validant les concepts décrits seront
incluses. NUTRICASUS tentera également de répondre aux questions les plus souvent posées
par rapport à la biologie nutritionnelle tant au niveau scientifique que logistique. Enfin
NUTRICASUS ouvre un espace pour vous permettre de vous exprimer librement par rapport à
la médecine nutritionnelle. En espérant que NUTRICASUS deviendra votre compagnon
incontournable dans votre pratique, nous vous réitérons nos souhaits de santé et de succès
pour 2006.
Nutritionnellement vôtre
Ellipsys S.A.
Le Cas Clinique du mois : Syndrome Métabolique
Histoire du Patient
Motif de la consultation:
Fatigue, excès de poids (+ 12 kilos en trois ans)
Anamnèse:
• Patient de 52 ans, marié et père de 3 enfants en bonne santé
• Fonctionnaire
• Se plaint de fatigue avec coups de pompe, sommeil difficile et surtout d'une
prise de poids importante depuis trois ans.
• Pas d'antécédents médicaux ou chirurgicaux significatifs
• Pas d'antécédents familiaux (notamment de diabète de type 2)
• Le médecin de famille lui a prescrit une biologie de contrôle avec notamment
une glycémie et insulinémie à jeun qui sont normales. Il conclut que le patient ne
présente aucune anomalie du métabolisme du glucose.
• On note un excès de triglycérides et de cholestérol LDL
• Le reste de la biologie est banale. Le médecin lui prescrit un régime
hypocalorique et un complexe vitaminé.
• Pas d'amélioration après deux mois.
Clinique
• Poids: 92 kilos Taille: 170 BMI: 31 (obésité)
• Examen clinique: patient adipeux, reste de l'examen sans particularité
Examens de Biologie fonctionnelle et Nutritionnelle prescrits:
• Hyperglycémie provoquée par voie orale (HGP0)
• Profil des acides gras
• Statut des défenses anti-oxydantes et stress oxydant
• Homocystéinémie
Histoire du Patient
Motif de la consultation:
Fatigue, excès de poids (+ 12 kilos en trois ans)
Anamnèse:
• Patient de 52 ans, marié et père de 3 enfants en bonne santé
• Fonctionnaire
• Se plaint de fatigue avec coups de pompe, sommeil difficile et surtout d'une
prise de poids importante depuis trois ans.
• Pas d'antécédents médicaux ou chirurgicaux significatifs
• Pas d'antécédents familiaux (notamment de diabète de type 2)
• Le médecin de famille lui a prescrit une biologie de contrôle avec notamment
une glycémie et insulinémie à jeun qui sont normales. Il conclut que le patient ne
présente aucune anomalie du métabolisme du glucose.
• On note un excès de triglycérides et de cholestérol LDL
• Le reste de la biologie est banale. Le médecin lui prescrit un régime
hypocalorique et un complexe vitaminé.
• Pas d'amélioration après deux mois.
Clinique
• Poids: 92 kilos Taille: 170 BMI: 31 (obésité)
• Examen clinique: patient adipeux, reste de l'examen sans particularité
Examens de Biologie fonctionnelle et Nutritionnelle prescrits:
• Hyperglycémie provoquée par voie orale (HGP0)
• Profil des acides gras
• Statut des défenses anti-oxydantes et stress oxydant
• Homocystéinémie
NUTRICASUS
Le Journal de la Médecine Nutritionnelle et Fonctionnelle Pratique
N°1, Janvier 2006 (à paraître tous les mois)
NUTRICASUS
Le Journal de la Médecine Nutritionnelle et Fonctionnelle Pratique
N°1, Janvier 2006 (à paraître tous les mois)

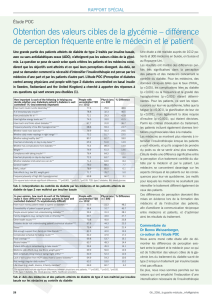
HGPO
Tps (min) Glycémie (mg/dl) Insulinémie (µU/ml)
0 97 (60-110) 11 (3-22)
30 120(100-180) 97 (18-79)
60 167 (100-180) 134 (29-88)
90 141 (100-150) 130 (27-80)
120 115 (70-120) 117 (22-79)
HGPO
Tps (min) Glycémie (mg/dl) Insulinémie (µU/ml)
0 97 (60-110) 11 (3-22)
30 120(100-180) 97 (18-79)
60 167 (100-180) 134 (29-88)
90 141 (100-150) 130 (27-80)
120 115 (70-120) 117 (22-79)
Le Cas Clinique du Mois : Résultats et Interprétation
Malgré une glycémie et insulinémie dans les limites
normales lorsque le patient est à jeun, les
concentrations d'insuline sont largement au dessus
de la normale pour pouvoir maintenir une glycémie
normale lorsque le patient consomme du glucose.
Cette situation définit le syndrome de résistance à
l'insuline, un des facteurs prépondérant du syndrome
métabolique
Malgré une glycémie et insulinémie dans les limites
normales lorsque le patient est à jeun, les
concentrations d'insuline sont largement au dessus
de la normale pour pouvoir maintenir une glycémie
normale lorsque le patient consomme du glucose.
Cette situation définit le syndrome de résistance à
l'insuline, un des facteurs prépondérant du syndrome
métabolique
Le profil d'acide gras indique un excès d'acides gras
saturés à courte chaîne (acide myristique) et d'acide
gras de type oméga-6. Il existe une carence nette en
acide gras oméga-3 notamment en acide
éicosapentaénoïque (EPA). L'enzyme delta-6-
désaturase ne fonctionne pas bien vu l'excès d'acide
alpha-linolénique (LNA-précurseur des oméga-3)
par rapport à l'EPA. Cette situation s'observe
notamment en cas d'hypersinsulinisme car l'excès
d'insuline inhibe la delta-6-désaturase
Le profil d'acide gras indique un excès d'acides gras
saturés à courte chaîne (acide myristique) et d'acide
gras de type oméga-6. Il existe une carence nette en
acide gras oméga-3 notamment en acide
éicosapentaénoïque (EPA). L'enzyme delta-6-
désaturase ne fonctionne pas bien vu l'excès d'acide
alpha-linolénique (LNA-précurseur des oméga-3)
par rapport à l'EPA. Cette situation s'observe
notamment en cas d'hypersinsulinisme car l'excès
d'insuline inhibe la delta-6-désaturase
PROFIL DES ACIDES GRAS
Carences en Vitamines A et E, taux de zinc et
sélenium trop bas avec excès de cuivre, taux de
ferritine basse et oxydation des LDL traduite par un
taux élevé d'anticorps anti-LDL oxydés.
Carences en Vitamines A et E, taux de zinc et
sélenium trop bas avec excès de cuivre, taux de
ferritine basse et oxydation des LDL traduite par un
taux élevé d'anticorps anti-LDL oxydés.
DEFENSES ANTI-OXYDANTES ET STRESS OXYDANT
200
175
150
125
100
75
50
25
%
Résultats du
p
atient en %:
VITA VITE AU SOD GPX SE ZN CU FERI TRF SAT OHDGACLDL
39 73 86 121 74 79 71 132 17 108 53 98 300
Valeurs de Référence en %:
68 74 59 78 67 77 77 74 19 70 67 82 50
132 126 141 122 133 123 123 126 181 130 133 118 150
Valeurs de Ré
f
érence Résultat normal Résultat hors des Valeurs de Ré
f
érence
1 1 2
39
73
86
121
74 79 71
132
17
108
53
98
300
HOMOCYSTEINEMIE: 17,6 µM Hyperhomocystéinémie (valeur santé doit être aux
alentours de 7µM)
Hyperhomocystéinémie (valeur santé doit être aux
alentours de 7µM)
Le Cas Clinique du Mois : Conclusions
Le patient souffre d'un syndrome de résistance à l'insuline avec déficience en acide gras oméga-3, excès d'acides gras
oméga 6 (pro-inflammatoires) et d'acide gras saturés. Il existe une activité diminuée de la delta-6-désaturase et un stress
oxydant lié entre autres à une carence en vitamines A et E et des taux trop bas de zinc et sélénium. Le patient souffre
également d'hyperhomocystéinémie. Une rééducation alimentaire basée sur la consommation d'aliments à index
glycémique bas, de poisson (3 x par semaine) et une complémentation comprenant des huiles oméga-3 (EPA et DHA), de
la vitamine E naturelle, un complexe anti-oxydant et un complexe B6/B9/B12 ont permis au patient de perdre de la masse
graisseuse et d'améliorer de manière remarquable son état clinique.
Le patient souffre d'un syndrome de résistance à l'insuline avec déficience en acide gras oméga-3, excès d'acides gras
oméga 6 (pro-inflammatoires) et d'acide gras saturés. Il existe une activité diminuée de la delta-6-désaturase et un stress
oxydant lié entre autres à une carence en vitamines A et E et des taux trop bas de zinc et sélénium. Le patient souffre
également d'hyperhomocystéinémie. Une rééducation alimentaire basée sur la consommation d'aliments à index
glycémique bas, de poisson (3 x par semaine) et une complémentation comprenant des huiles oméga-3 (EPA et DHA), de
la vitamine E naturelle, un complexe anti-oxydant et un complexe B6/B9/B12 ont permis au patient de perdre de la masse
graisseuse et d'améliorer de manière remarquable son état clinique.
NUTRICASUS
Le Journal de la Médecine Nutritionnelle et Fonctionnelle Pratique
NUTRICASUS
Le Journal de la Médecine Nutritionnelle et Fonctionnelle Pratique

Syndrome Métabolique : Références Scientifiques Choisies
n-3 long-chain polyunsaturated fatty acids in type 2 diabetes: a
review.
Nettleton JA, Katz R.
J Am Diet Assoc. 2005 Mar;105(3):428-40.
Historically, epidemiologic studies have reported a lower prevalence
of impaired glucose tolerance and type 2 diabetes in populations
consuming large amounts of the n-3 long-chain polyunsaturated fatty
acids (n-3 LC-PUFAs) found mainly in fish. Controlled clinical
studies have shown that consumption of n-3 LC-PUFAs has
cardioprotective effects in persons with type 2 diabetes without
adverse effects on glucose control and insulin activity. Benefits
include lower risk of primary cardiac arrest; reduced cardiovascular
mortality, particularly sudden cardiac death; reduced triglyceride
levels; increased high-density lipoprotein levels; improved endothelial
function; reduced platelet aggregability; and lower blood pressure.
These favorable effects outweigh the modest increase in low-density
lipoprotein levels that may result from increased n-3 LC-PUFA
intake. Preliminary evidence suggests increased consumption of n-3
LC-PUFAs with reduced intake of saturated fat may reduce the risk of
conversion from impaired glucose tolerance to type 2 diabetes in
overweight persons. Reported improvements in hemostasis, slower
progression of artery narrowing, albuminuria, subclinical
inflammation, oxidative stress, and obesity require additional
confirmation. Expected health benefits and public health implications
of consuming 1 to 2 g/day n-3 LC-PUFA as part of lifestyle
modification in insulin resistance and type 2 diabetes are discussed.
n-3 long-chain polyunsaturated fatty acids in type 2 diabetes: a
review.
Nettleton JA, Katz R.
J Am Diet Assoc. 2005 Mar;105(3):428-40.
Historically, epidemiologic studies have reported a lower prevalence
of impaired glucose tolerance and type 2 diabetes in populations
consuming large amounts of the n-3 long-chain polyunsaturated fatty
acids (n-3 LC-PUFAs) found mainly in fish. Controlled clinical
studies have shown that consumption of n-3 LC-PUFAs has
cardioprotective effects in persons with type 2 diabetes without
adverse effects on glucose control and insulin activity. Benefits
include lower risk of primary cardiac arrest; reduced cardiovascular
mortality, particularly sudden cardiac death; reduced triglyceride
levels; increased high-density lipoprotein levels; improved endothelial
function; reduced platelet aggregability; and lower blood pressure.
These favorable effects outweigh the modest increase in low-density
lipoprotein levels that may result from increased n-3 LC-PUFA
intake. Preliminary evidence suggests increased consumption of n-3
LC-PUFAs with reduced intake of saturated fat may reduce the risk of
conversion from impaired glucose tolerance to type 2 diabetes in
overweight persons. Reported improvements in hemostasis, slower
progression of artery narrowing, albuminuria, subclinical
inflammation, oxidative stress, and obesity require additional
confirmation. Expected health benefits and public health implications
of consuming 1 to 2 g/day n-3 LC-PUFA as part of lifestyle
modification in insulin resistance and type 2 diabetes are discussed.
Syndrome X: medical nutrition therapy.
Roberts K, Dunn K, Jean SK, Lardinois CK
Nutr Rev 2000 May;58(5):154-60
A significant number of Americans are at risk for developing a
condition of insulin resistance termed Syndrome X. Dyslipidemia,
resistance to insulin, obesity, and blood pressure elevation--the deadly
quartet--describe Syndrome X, which increases atherogenic risk and
contributes to coronary artery disease. Lifestyle factors such as
overeating and physical inactivity play a pivotal role in Syndrome X.
This deadly duet has been aptly coined "hyperactive fork" and
"hypoactive foot," respectively. In addition, emerging evidence
suggests that certain nutrients may help protect against Syndrome X.
This review provides a brief discussion of diet and lifestyle factors
related to Syndrome X.
Syndrome X: medical nutrition therapy.
Roberts K, Dunn K, Jean SK, Lardinois CK
Nutr Rev 2000 May;58(5):154-60
A significant number of Americans are at risk for developing a
condition of insulin resistance termed Syndrome X. Dyslipidemia,
resistance to insulin, obesity, and blood pressure elevation--the deadly
quartet--describe Syndrome X, which increases atherogenic risk and
contributes to coronary artery disease. Lifestyle factors such as
overeating and physical inactivity play a pivotal role in Syndrome X.
This deadly duet has been aptly coined "hyperactive fork" and
"hypoactive foot," respectively. In addition, emerging evidence
suggests that certain nutrients may help protect against Syndrome X.
This review provides a brief discussion of diet and lifestyle factors
related to Syndrome X.
Hyperhomocysteinemia correlates with insulin resistance and low-grade systemic inflammation in obese prepubertal children.
Martos R, Valle M, Morales R, Canete R, Gavilan MI, Sanchez-Margalet V.
Metabolism. 2006 Jan;55(1):72-7. Obesity is an independent risk factor for the development of cardiovascular disease frequently associated
with hypertension, dyslipemia, diabetes, and insulin resistance. Higher homocysteine (Hcy) levels are observed in the hyperinsulinemic obese
adults and suggest that Hcy could play a role in the higher risk of cardiovascular disease in obesity. We analyzed total Hcy levels in obese
prepubertal children and their possible association with both metabolic syndrome and various inflammatory biomarkers and leptin. We studied
43 obese children (aged 6-9 years) and an equal number of nonobese children, paired by age and sex. The obese subjects presented
significantly elevated values for insulin (P = .003), C-reactive protein (P = .033), and leptin (P < .001). No significant differences were found
in Hcy levels between the obese and nonobese children. However, Hcy concentration was significantly higher in the hyperinsulinemic obese
children than in the normoinsulinemic group (P = .002). Using multivariant regression analysis, in the obese group, corrected for age and sex,
the homeostasis model assessment for insulin resistance (P partial = .001) and leptin (P partial = .02) are independent predictive factors for
Hcy. In the control group, corrected for age and sex, the homeostasis model assessment for insulin resistance (P partial = .005) and leptin (P
partial = .031) also are independent predictive factor for Hcy. Increased plasma Hcy, particularly in hyperinsulinemic obese children, may be
causally involved in the pathogenesis of atherosclerosis and/or cardiovascular disease, both of which are common in obesity.
Hyperhomocysteinemia correlates with insulin resistance and low-grade systemic inflammation in obese prepubertal children.
Martos R, Valle M, Morales R, Canete R, Gavilan MI, Sanchez-Margalet V.
Metabolism. 2006 Jan;55(1):72-7. Obesity is an independent risk factor for the development of cardiovascular disease frequently associated
with hypertension, dyslipemia, diabetes, and insulin resistance. Higher homocysteine (Hcy) levels are observed in the hyperinsulinemic obese
adults and suggest that Hcy could play a role in the higher risk of cardiovascular disease in obesity. We analyzed total Hcy levels in obese
prepubertal children and their possible association with both metabolic syndrome and various inflammatory biomarkers and leptin. We studied
43 obese children (aged 6-9 years) and an equal number of nonobese children, paired by age and sex. The obese subjects presented
significantly elevated values for insulin (P = .003), C-reactive protein (P = .033), and leptin (P < .001). No significant differences were found
in Hcy levels between the obese and nonobese children. However, Hcy concentration was significantly higher in the hyperinsulinemic obese
children than in the normoinsulinemic group (P = .002). Using multivariant regression analysis, in the obese group, corrected for age and sex,
the homeostasis model assessment for insulin resistance (P partial = .001) and leptin (P partial = .02) are independent predictive factors for
Hcy. In the control group, corrected for age and sex, the homeostasis model assessment for insulin resistance (P partial = .005) and leptin (P
partial = .031) also are independent predictive factor for Hcy. Increased plasma Hcy, particularly in hyperinsulinemic obese children, may be
causally involved in the pathogenesis of atherosclerosis and/or cardiovascular disease, both of which are common in obesity.
Proposed mechanisms for the induction of insulin resistance
by oxidative stress.
Bloch-Damti A, Bashan N.
Antioxid Redox Signal. 2005 Nov-Dec;7(11-12):1553-67.
In diabetes (type 1 and type 2), increased flux of free fatty acids
and glucose is associated with increased mitochondrial reactive
oxygen species (ROS) production and, as a consequence,
increased oxidative stress. ROS have been shown to activate
various cellular stress-sensitive pathways, which can interfere
with cellular signaling pathways. Exposure of different cell lines
to micromolar concentrations of hydrogen peroxide leads to the
activation of stress kinases such as c-Jun N-terminal kinase, p38,
IkappaB kinase, and extracellular receptor kinase 1/2. This
activation is accompanied by a down-regulation of the cellular
response to insulin, leading to a reduced ability of insulin to
promote glucose uptake, and glycogen and protein synthesis. The
mechanisms leading to this down-regulation in oxidized cells are
complicated, involving increased serine/threonine
phosphorylation of insulin receptor substrate-1 (IRS1), impaired
insulin-stimulated redistribution of IRS1 and
phosphatidylinositol-kinase between cytosol and low-density
microsomal fraction, followed by a reduced protein kinase-B
phosphorylation and GLUT4 translocation to the plasma
membrane. In addition, prolonged exposure to ROS affects
transcription of glucose transporters: whereas the level of
GLUT1 is increased, GLUT4 level is reduced. As can be
expected, administration of antioxidants such as lipoic acid in
oxidized cells, in animal models of diabetes, and in type 2
diabetes shows improved insulin sensitivity. Thus, oxidative
stress is presently accepted as a likely causative factor in the
development of insulin resistance.
Proposed mechanisms for the induction of insulin resistance
by oxidative stress.
Bloch-Damti A, Bashan N.
Antioxid Redox Signal. 2005 Nov-Dec;7(11-12):1553-67.
In diabetes (type 1 and type 2), increased flux of free fatty acids
and glucose is associated with increased mitochondrial reactive
oxygen species (ROS) production and, as a consequence,
increased oxidative stress. ROS have been shown to activate
various cellular stress-sensitive pathways, which can interfere
with cellular signaling pathways. Exposure of different cell lines
to micromolar concentrations of hydrogen peroxide leads to the
activation of stress kinases such as c-Jun N-terminal kinase, p38,
IkappaB kinase, and extracellular receptor kinase 1/2. This
activation is accompanied by a down-regulation of the cellular
response to insulin, leading to a reduced ability of insulin to
promote glucose uptake, and glycogen and protein synthesis. The
mechanisms leading to this down-regulation in oxidized cells are
complicated, involving increased serine/threonine
phosphorylation of insulin receptor substrate-1 (IRS1), impaired
insulin-stimulated redistribution of IRS1 and
phosphatidylinositol-kinase between cytosol and low-density
microsomal fraction, followed by a reduced protein kinase-B
phosphorylation and GLUT4 translocation to the plasma
membrane. In addition, prolonged exposure to ROS affects
transcription of glucose transporters: whereas the level of
GLUT1 is increased, GLUT4 level is reduced. As can be
expected, administration of antioxidants such as lipoic acid in
oxidized cells, in animal models of diabetes, and in type 2
diabetes shows improved insulin sensitivity. Thus, oxidative
stress is presently accepted as a likely causative factor in the
development of insulin resistance.
L'implication du syndrome de résistance à l'insuline dans
le développement du diabète de type II et des ses
complications n'est plus à démontrer. De nombreuses
études expérimentales et cliniques ont également montré
un rôle majeur de la carence en acides gras oméga-3 et
de l'excès d'acides gras saturés, du stress oxydant
notamment au niveau des membranes cellulaires ansi que
des troubles des réactions de méthylation traduits par une
hyperhomocystéinémie.
L'implication du syndrome de résistance à l'insuline dans
le développement du diabète de type II et des ses
complications n'est plus à démontrer. De nombreuses
études expérimentales et cliniques ont également montré
un rôle majeur de la carence en acides gras oméga-3 et
de l'excès d'acides gras saturés, du stress oxydant
notamment au niveau des membranes cellulaires ansi que
des troubles des réactions de méthylation traduits par une
hyperhomocystéinémie.
NUTRICASUS
Le Journal de la Médecine Nutritionnelle et Fonctionnelle Pratique
NUTRICASUS
Le Journal de la Médecine Nutritionnelle et Fonctionnelle Pratique

Questions fréquemment posées :
•Qui envoie les graphiques, commentaires, conseils micronutritionnels et nutritionnels ?
ÎLa société Ellipsys.
•Qui réalise les analyses de biologie ?
ÎLe laboratoire Roman Païs.
•A qui sont destinés graphiques, commentaires, conseils micronutritionnels et
nutritionnels ?
ÎAu praticien.
•Comment puis-je faire pour recevoir le matériel nécessaire aux bilans nutritionnels, quels
sont les délais ?
ÎTéléphonez au +32 67 645 200 afin de passer la commande. 100% des commandes
sont fabriquées dans les 5 jours ouvrables. La livraison s’effectue par un chauffeur du
laboratoire en Belgique et par transporteur à l’étranger.
•Comment mes patients peuvent-ils régler la facture du laboratoire ?
ÎPar carte de crédit (Visa, Mastercard), par virement bancaire, en numéraire ou par
chèque.
Questions fréquemment posées :
•Qui envoie les graphiques, commentaires, conseils micronutritionnels et nutritionnels ?
ÎLa société Ellipsys.
•Qui réalise les analyses de biologie ?
ÎLe laboratoire Roman Païs.
•A qui sont destinés graphiques, commentaires, conseils micronutritionnels et
nutritionnels ?
ÎAu praticien.
•Comment puis-je faire pour recevoir le matériel nécessaire aux bilans nutritionnels, quels
sont les délais ?
ÎTéléphonez au +32 67 645 200 afin de passer la commande. 100% des commandes
sont fabriquées dans les 5 jours ouvrables. La livraison s’effectue par un chauffeur du
laboratoire en Belgique et par transporteur à l’étranger.
•Comment mes patients peuvent-ils régler la facture du laboratoire ?
ÎPar carte de crédit (Visa, Mastercard), par virement bancaire, en numéraire ou par
chèque.
Agenda :
• 28/03 Bruxelles
• 29/03 Liège
• 13/06 Bruxelles
• 14/06 Liège
• 22-23-24/06 Valencia (Espagne) « La Calderona »
Le programme détaillé sera présenté dans le prochain Nutricasus.
Agenda :
• 28/03 Bruxelles
• 29/03 Liège
• 13/06 Bruxelles
• 14/06 Liège
• 22-23-24/06 Valencia (Espagne) « La Calderona »
Le programme détaillé sera présenté dans le prochain Nutricasus.
Vos commentaires (A envoyer par fax au +32 67 217 068 ou par e-mail à [email protected])
Cet espace est réservé à vos commentaires et remarques concernant vos expériences par rapport à
l'utilisation des bilans nutritionnels dans votre pratique. L'objectif est de librement donner votre point de vue
avec comme objectif de rendre encore plus utile et plus efficace le service que le laboratoire Roman Païs
vous offre.
Vos commentaires (A envoyer par fax au +32 67 217 068 ou par e-mail à [email protected])
Cet espace est réservé à vos commentaires et remarques concernant vos expériences par rapport à
l'utilisation des bilans nutritionnels dans votre pratique. L'objectif est de librement donner votre point de vue
avec comme objectif de rendre encore plus utile et plus efficace le service que le laboratoire Roman Païs
vous offre.
Editeur responsable
Ellipsys S.A.
Green Alley Office Park
70 Rue du Panier Vert
B-1400 Nivelles
Belgique Nutricasus est rédigé par des experts dans le domaine de la nutrition
info@ellipsys.be sous la supervision du comité scientifique du laboratoire Roman Païs.
Editeur responsable
Ellipsys S.A.
Green Alley Office Park
70 Rue du Panier Vert
B-1400 Nivelles
Belgique Nutricasus est rédigé par des experts dans le domaine de la nutrition
info@ellipsys.be sous la supervision du comité scientifique du laboratoire Roman Païs.
NUTRICASUS
Le Journal de la Médecine Nutritionnelle et Fonctionnelle Pratique
NUTRICASUS
Le Journal de la Médecine Nutritionnelle et Fonctionnelle Pratique
1
/
4
100%