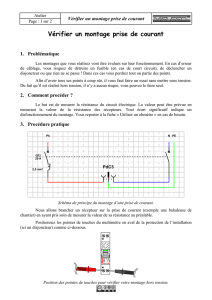
Les médicaments utilisés dans la maladie d`Alzheimer

Les$médicaments$utilisés$dans$la$maladie$d’Alzheimer$
!
!
La$pathologie$:$
" #$$%&'()*!*%+,)-./.*.,0'(1%!&)*-+(20*'!3,)/,%22(1%4%*'!%'!
(,,.1%,2(56%4%*'!7!60!3%,'%!-%!4.4)(,%!804*.2(%9!%'!-%2!$)*&'()*%2!
&)/*('(1%2!:!
o #3;02(%!:!3%,'%!-+!60*/0/%!
o #3,0<(%!:!3%,'%!-%!60!2',0'./(%!-%2!4)+1%4%*'2!
o #/*)2(%!:!3%,'%!-%!60!,%&)**0(220*&%!1(2+%66%!
" =*%!3,.)&&+30'()*!40>%+,%!-%!20*'.!3+56(?+%!
o @*&(-%*&%!ABB!BBB!7!CBB!BBB!%*!D,0*&%!
o E(%(66(22%4%*'!-%!60!3)3+60'()*!
o F,.106%*&%!:!CG!-%2!36+2!-%!HC!0*2I!JBG!-%2!36+2!-%!KB!0*2!
o L!%*!#MNI!OPA!*)*!-.&60,.2!
!
Physiopathologie$:$
" Q'()6)/(%!
o M0!4060-(%!,.2+6'%!-%!J!'R3%2!-%!6.2()*2!-0*2!6%!&%,1%0+!
40(*'%*0*'!5(%*!&0,0&'.,(2.%2!
! F60?+%2!2.*(6%2!:!-.3S'2!0*),40+<!%*',%!6%2!*%+,)*%2!-T+*%!
3,)'.(*%I!5%'0!04R6)U-%I!$,0/4%*'!-T+*%!3,)'.(*%!-%!
4%45,0*%!8#FF!Amyloid(Precursor(ProteinI!!30,!0&'()*!-%2!
063;0!%'!/0440!2.&,.'02%29!-)*&!-%2',+&'()*!-%2!*%+,)*%2!
01)(2(*0*'2!
! N./.*.,%2&%*&%!*%+,)$(5,(660(,%!:!0&&+4+60'()*!-%!
$(604%*'2!0*),40+<!&)*2'('+.2!-%!3,)'.(*%2!'0+!
;R3%,3;)23;),R6.%2!-)*&!(*'%,,+3'()*!-+!$6+<!)+!-+!
',0*23),'!0<)*06!84),'!*%+,)*06%9!%'!-)*&!3%,'%!-%!
4.4)(,%!%'!-.4%*&%V!
!
Prise$en$charge$thérapeutique$
Les$traitements$actuels$:$
F02!-%!',0('%4%*'!&+,0'($!WW!
" @*;(5('%+,!-%!6T#&;Q!:!6(4('%!6%!40*?+%!-T#&;!-0*2!6%!&%,1%0+!:!
o N)*.3.X(6!8#,(&%3'Y9!
!
!
!
!
!
!
!

o Z(102'(/4(*%!8Q[QM\]Y9!
!
o ^060*'04(*%!8Z%4(*R6Y9!
!
!
" #*'0/)*(2'%!*)*!&)43.'('($!-+!,.&%3'%+,!0+!]_N#!
o _%40*'(*%!8Q`@[#Y9!!
!
!
I. Les$inhibiteurs$de$l’AchE$
0V \,(/(*%!-%!60!4060-(%!-T#6X;%(4%,!
!
" F%,'%!-%!6T0&'(1('.!&;)6(*%,/(?+%!:!
o `0(22%!-%!60!-%*2('.!-%2!,.&%3'%+,2!*(&)'(*(?+%2!7!6T#&;!
o `0(22%!-%!60!3,)-+&'()*!-T#&;!80&'(1('.!&;)6(*%"0&.'R6',0*2$.,02%9!
o #+/4%*'0'()*!-%2!%*XR4%2!6R20*'!6T#&;!
! #33,)&;%!';.,03%+'(?+%!:!,.'056(22%4%*'!-+!'0+<!-T#&;!
5V MT0&.'R6&;)6(*%2'.,02%!
" #&;!:!]_!-%!6)&06(20'()*!&%*',06%!%'!3.,(3;.,(?+%!
" N./,0-0'()*!30,!J!&;)6(*%2'.,02%2!
o #&;Q!:!a]b!
o `+'R,(?+%!b;)6(*%2'.,02%2!:!a]F!
" @*;(5('%+,2!&%*',0+<I!2.6%&'($2I!c&)43.'('($2!%'!,.1%,2(56%2!
!
!
&V N)*.3.X(6!#Z@bQFd!Y!
!
" F(3.,(-(*%!
" Je4%!0,,(1.!2+,!6%!40,&;.!03,%2!d0&,(*%!8b\^]Q[!,%'(,.9!
" Z%2'%!,.$.,%*&%!
" @*;(5('%+,!*)*!&)43.'('($I!,.1%,2(56%!
" #$$(*('.!#&;QP`+&;Q!f!CBB!7!OBBB!
" b3!C"OB!4/!
" @*2'0+,0'()*!3,)/,%22(1%!-+!',0('%4%*'!

" Q@!30,'(&+6(%,!:!(*2)4*(%!:!-.&06%,!6T;%+,%!-%!3,(2%!%*!$(*!-T03,e2"4(-(!0+!
6(%+!-+!&)+&;%,V!
!
-V Z(102'(/4(*%!Q[QM\]Y!
!
" b)44%,&(06(2.!03,e2!6T#Z@bQFd!
" b0,5040'%!&)44%!-T0+',%2!(*;(5('%+,2!-%!&;)6(*%2'.,02%2!
83;R2)2'(/4(*%P(*2%&'(&(-%29!
" @*;(5('%+,!&)43.'('($!32%+-)"(,,.1%,2(56%!
" #$$(*('.!#&;Q!f!`b;Q!
" ^.6+6%2!OIC"A"gIC"H4/I!2)6!5+1I!30'&;!
" @*2'0+,0'()*!-+!',0('%4%*'!3,)/,%22(1%!20+$!3)+,!30'&;!
!
%V ^060*'04(*%!ZQ_@]hMY!
!
" #6&06)U-%!
" #&'(1('.!:!
o @*;(5('()*!2.6%&'(1%!-%!6T#&;Q!
o _)-+60'()*!066)2'.,(?+%!-%2!,.&%3'%+,2!*(&)'(*(?+%2!8*#&;Z9!
" i(2'),(?+%!:!
o OjCB!:!-.&)+1%,'%!-%!60!/060*'04(*%!-+!3%,&%"*%(/%!
o OjHO!:!+'(6(20'()*!-0*2!6%2!-)+6%+,2!*%,1%+2%2!
o OjjB!:!&)*$(,40'()*!-%!6T(*;(5('()*!-%!6T#&;QV!Q'+-%2!%'!%220(2!
&6(*(?+%2!-0*2!60!_#!
o JBBO!:!4(2%!2+,!6%!40,&;.!-%!Z.4(*R6!%*!D,0*&%V!
!
Effets$indésirables$des$inhibiteurs$de$l’AchE$
" Q@!b;)6(*%,/(?+%2!:!
o ]0+2.%2I!-(0,,;.%2!
o `,)*&;('%2!02';40'($),4%2!
o `,0-R&0,-(%!
o N)+6%+,2!4+2&+60(,%2I!&,043%2!
o d,%456%4%*'2!
" F)+,!6(4('%,!&%2!%$$%'2!)*!3%+'!$,0&'()**%,!6%2!-)2%2!%'!3,%*-,%!3%*-0*'!
6%2!,%302!82+,')+'!3)+,!,(102'(/4(*%!%'!/060*'04(*%9!
!
$
II. Antagoniste$du$récepteur$au$NMDA$
0V i(2'),(?+%!!
!
OjHA!:!3,%4(e,%2!2R*';e2%!-+!OIA"-(4.';R6"C"04(*)0-040*'0*%V!
" #*06)/+?%!6(3)3;(6%!-%!6T0-040*'0*%!
" #,&;('%&'+,%!&)430&'%!%'!;0+'%4%*'!2R4.',(?+%!
OjHg!:!#40*'0-(*%!0*'("1(,06%!8/,(33%9!
" \52%,10'()*2!&6(*(?+%2!:!0&'(1('.!2%&)*-0(,%!2+,!6%!&%,1%0+!-%2!3%,2)**%2!
k/.%2!!
OjlJ!:!',0('%4%*'!-%!60!4060-(%!-%!F0,m(*2)*!
OjlB2!:!Q'+-%2!2+,!60!_.40*'(*%!

OjKj!:!6%2!0*'0/)*(2'%2!-+!,.&%3'%+,!]_N#!8]"4.';R6"N"0230,'0'%9!$0&(6('%*'!
6T033,%*'(220/%!
OjjC!:!_.40*'(*%!&)44%!0/%*'!';.,03%+'(?+%!3)'%*'(%6!-%!60!4060-(%!
-T#6X;%(4%,!
JBBJ!:!_.40*'(*%!033,)+1.%!%*!Q+,)3%!3)+,!6%!',0('%4%*'!-%!60!4060-(%!
-T#6X;%(4%,!4)-.,.%!7!2.1e,%!:!Q`@[#!&3!OB4/I!2)6!5+1I!A!306(%,2!8(*2'0+,0'()*!
-+!',0('%4%*'!3,)/,%22(1%9!
!
5V aR*';e2%!-%!60!_.40*'(*%!
!
!
&V @*$6+%*&%!-+!/6+'040'%!
!
" F%3'(-%!#5%'0!O"gJ!2T0&&+4+6%!:!n^6+o!0+/4%*'%!-0*2!60!$%*'%!2R*03'(?+%!
8-.2.?+(6(5,%!6(5.,0'()*P&03'+,%9!
" Q<&(')')<(&('.!:!^6+!0/)*(2'%!-+!,.&%3'%+,!]_N#I!*.$02'%!2(!',)3!/,0*-%!
?+0*'('.!820'+,0'()*9!
" iR3%,0&'(10'()*!-+!,.&%3'%+,!
" Z.&%3'%+,!&0*06!56)?+.!)+1%,'!36+2!-%!$%,4%'+,%I!30,!_/Jp!
" _.40*'(*%!2%!$(<%!2+,!6%!2('%!-%!$(<0'()*!-+!_/Jp!8*)*!&)43.'('($!01%&!6%!
^6+9!,%56)?+%!6%!,.&%3'%+,!
" D(<0'()*!+*(?+%4%*'!2(!,.&%3'%+,!3,.0&'(1.!8)+1%,'9!%'!-)*&!+*(?+%4%*'!
2(!30';)6)/(%!
!
-V M%!,.&%3'%+,!]_N#!
!
" Z.&%3'%+,!&0*06!
" i0+'%!3%,4.05(6('.!1(2!7!1(2!-+!b06&(+4!80&&+4+60'()*!(*',0&%66+60(,%9!
" b0*06!56)?+.!)+1%,'!30,!_0/*.2(+4!',e2!$),'%4%*'!1)6'0/%!-.3%*-0*'!
!
%V Q$$%'2!(*-.2(,056%2!
" @*2)4*(%2!
" E%,'(/%2!
" _0+<!-%!'q'%!
" i066+&(*0'()*2!
" a+,1%(66%,!60!$)*&'()*!,.*06%!
!
$V @*'%,0&'()*2!4.-(&04%*'%+2%2!
" #/)*(2'%2!-)304(*%,/(?+%2!
" #*'(&;)6(*%,/(?+%2!

$
III. RPDO$(pour$les$4)$
!
" F,%2&,(3'()*!(*('(06%!0**+%66%!,.2%,1.%2!:!*%+,)6)/(%I!32R&;(0',(%I!/.,(0',(%!
" Z%*)+1%66%4%*'!30,!')+'!3,%2&,(3'%+,!
" F,%2&,(3'()*!(*('(06%!7!3,.2%*'%,!0+!3;0,40&(%*!7!&;0?+%!,%*)+1%66%4%*'!
$
IV. Le$traitement$symptomatique$
!
" @aZa!8(*;(5('%+,2!2.6%&'($2!-%!60!,%&03'+,%!-%!60!2.,)')*(*%9!&)*',%!
',(2'%22%I!(,,('05(6('.I!0*<(.'.I!;)2'(6('.I!(-.%2!-.6(,0*'%2r!
" #*'(32R&;)'(?+%2!0'R3(?+%2!&)*',%!6T0/('0'()*I!6T0/,%22(1('.I!6%2!
;066+&(*0'()*2I!(-.%2!-.6(,0*'%2r!
" #*'(.3(6%3'(?+%2!';R4),./+60'%+,2!&)*',%!6T0/('0'()*I!6T(,,('05(6('.!%'!
6T;)2'(6('.r!
!
!
Conclusion$:$
" F,)56e4%!:!.106+0'()*!-%!6T%$$(&0&('.!-+!',0('%4%*'r!
" d,0('%4%*'2!2R43')40'(?+%2!?+(!*T0,,q'%!302!60!4060-(%!
o MT#&;Q!:!2+,!6%2!$),4%2!6./e,%2!%'!4)-.,.4%*'!2.1e,%2!
o @*;(5('%+,!-+!,.&%3'%+,!]_N#!s!$),4%2!4)-.,.%2!7!2.1e,%2!
" ]%!302!)+56(%,!:!3,(2%!%*!&;0,/%!*)*!4.-(&04%*'%+2%!82'(4+60'()*!
&)/*('(1%I!,..-+&0'()*!),';)3;)*(?+%!%'!32R&;)4)',(&%r9!0(-%!7!60!
3%,2)**%!%'!2)+'(%*!0+<!$04(66%2V!
!
1
/
5
100%