Fluid Phase Equilibria, 4 (1980) 125-136 125
0 Elsevier Scientific Publishing Company, Amsterdam - Printed in The Netherlands
EXTRACTION OF TETRAHYDROFURAN FROM AQUEOUS
SOLUTIONS. TERNARY LIQUID EQUILIBRIA WITH CHLORO-
METHANES AND CHLOROETHANES AS SOLVENTS
JOSE COCA * , RAMONA M. DIAZ and CARMEN PAZOS
Department of Chemical Engineering, University of Oviedo, Oviedo (Spain)
(Received March 13th, 1979; accepted July 30th, 1979)
ABSTRACT
Coca, J., Diaz, R.M. and Pazos, C., 1980. Extraction of tetrahydrofuran from aqueous
solutions. Ternary liquid equilibria with chloromethanes and chloroethanes as solvents.
Fluid Phase Equilibria, 4: 125-136.
Liquid-liquid equilibrium data at 25OC for tetrahydrofuran-water with methylene
chloride, chloroform, carbon tetrachloride, 1,2-dichloroethane and 1,1,2-trichloroethane
as solvents have been measured. The tie-line data have been correlated by the methods of
Hand, Othmer-Tobias and Bachman; plait points have been estimated following the
methods of Treybal et al. and Mato and Bueno. All the solvents studied present isopicnic
compositions and selectivity is very similar for all of them.
INTRODUCTION
The separation of tetrahydrofuran (THF) from aqueous solutions is of par-
ticular interest due to the significant demand for THF as a solvent for extrac-
tion purposes and chemical reactions. THF-water mixtures show a closed-
loop region of limited miscibility over the temperature range of ‘71.8 to
137.1’C that might be useful to enrich the mixture in THF (Matoug, Novak,
Sobr and Pick, 1972). Liquid-liquid extraction can be used to recover THF
from the resulting phases and also from a mixture which has a composition
(of THF) lower than 25% in weight, in which phase separation does not
occur. THF also forms an azeotropic mixture with water (Cigna and Sebas-
tiani, 1964) and in this case as well liquid-liquid extraction can be a suitable
method of recovering THF by distillation.
In this work liquid-liquid equilibrium data for the ternary systems THF-
water with methylene chloride, chloroform, carbon tetrachloride, 1,2-di-
chloroethane and 1,1,2-trichloroethane as solvents are presented at 25°C.
Solvent selection was made on basis of Ewell’s criteria (1944).
l To whom correspondence should be addressed.

126
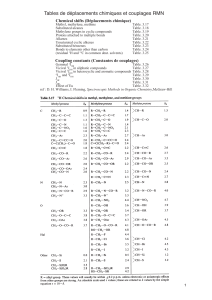
TABLE 1
Physical properties of the chemicals
Chemical Property Exptl. Lit.
Tetrahydrofuran do 0.8875 0.8880 s
o.8892"
ng” 1.4072 1.4073 a
Methylene chloride ds” 1.3266 1.3264 b
n”D” 1.4242 1.4242 b
Chloroform 20 d4 1.4834 1.4832 b
n$O 1.4459 1.4459 b
Carbon tetrachloride 20 d4 1.5943 1.5940 b
r&o 1.4603 1.4601 b
1,2-Dichloroethane 4 20 1.2530 1.2531 b
n&O 1.4449 1.4448 b
1,1,2-Trichloroethane 4 20 1.4401 1.4397 b
r&o 1.4711 1.4714 b
a Data taken from “Solvents Guide” (1963).
b Data taken from “Handbook of Chemistry and Physics” (1971-72).
I
l
i
Ih I
-7?3-
Fig. 1. Schematic view of the titration assembly.
Fig. 2. Densities and refractive indices for the system THF-water-1,1,2-trichloroethane.
A,O,at24”C;A,o,at 26°C.

127
EXPERIMENTAL
Materials
Tetrahydrofuran, chloroform and 1,1,2+ichloroethane were supplied by
Fluka and methylene chloride, carbon tetrachloride and 1,bdichloroethane
by Probus. All chemicals were further purified by distillation in a heli-pack-
ing column. Distilled water was used in all the systems. The physical proper-
ties of the chemicals are given in Table 1.
Apparatus and operations
The mutual solubility data at 25°C were determined by the method as
described by Othmer et al. (1941); homogeneous binary mixtures were
titrated with the third component until the onset of turbidity. The titration
was carried out in a thermostated erlenmeyer flask, Fig. 1, with a silicone
gum stopper to prevent evaporation, the third component being added
through a needle inserted into the stopper. Under these conditions, at 25°C
for the most volatile solvent used in this work evaporation losses were of the
order of 0.23% in weight for a one hour period. Taking into account that
titrations with this solvent did not last more than 30 min and that it was
mixed with higher boiling point components, evaporation losses can be con-
sidered negligible.
During titration, the mixture was stirred with a magnetic stirrer. Mutual
solubilities of the solvent-water systems are given in Table 2 and also
TABLE 2
Mutual solubilities of the solvent-water systems
Chemical Solubility Exptl. = Lit.
Methylene chloride
Chloroform
Carbon tetrachloride
1,2-Dichloroethane
1,1,2-Trichloroethane
in water
water in
in water
water in
in water
water in
in water
water in
in water
water in
1.37% (25’C)
0.18% (25°C)
1.19% (25°C)
0.08% (25°C)
0.07% (25°C)
0.01% (25°C)
1.10% (25°C)
0.18% (25°C)
0.50% (25’C)
0.085% (2VC)
1.32 g/100 g (25°C) a
0.198 g/100 g (25°C) s
0.822 g/100 g (20°C) *
0.072 g/100 g (23°C) a
0.077 g/100 ml (25°C) a
0.010 g/100 ml (24°C) a
0.81% (20°C) a.c
0.15% (20°C) a&
0.45% (2OOC) b*c
0.05% (20°C) b*c
s Data taken from “Organic Solvents” (1955).
b Data taken from “Solvents Guide” (1963).
c Weight percent.

THF
WATER SOLVENT
10 20 30 LO 50 60 70 80 90
Fig. 3. Mutual solubility curves for THF-water-solvent systems at 25” C. n , methylene
chloride; +, chloroform; x , carbon tetrachloride; 0, l,Z-dichloroethane; A, 1,1,2-trichloro-
ethane.
TABLE 3
Mutual solubility and tie-line data for THF-water(W jmethylene chloride(MC) at 25°C
(values expressed in weight percent)
Mutual solubility data
THF W MC THF W MC
0.00 0.18 99.82 72.35 21.41 6.24
18.23 0.54 81.23 68.70 26.17 5.13
31.93 1.19 66.89 65.48 30.08 4.44
43.37 1.91 54.72 57.49 39.38 3.12
55.64 2.89 41.47 50.11 47.78 2.11
63.74 4.07 32.19 42.27 56.31 1.42
68.35 5.09 26.56 34.09 64.93 0.98
72.10 6.34 21.66 26.33 72.77 0.90
75.66 8.47 15.85 18.86 80.32 0.82
76.64 10.33 13.03 11.04 87.84 1.12
76.55 13.79 9.66 3.83 94.87 1.30
74.87 17.32 7.81 0.00 98.63 1.37
Tie-line data
Aqueous phase
THF W MC
Organic phase
THF W MC
48.55 49.39 2.06 71.94 21.88 6.18
30.80 68.23 0.97 76.50 10.05 13.45
25.12 74.08 0.80 73.50 6.95 19.55
17.80 81.36 0.84 64.25 4.32 31.43
13.22 85.86 0.92 53.82 2.66 43.52
8.39 90.52 1.09 42.04 1.59 56.37
2.20 96.61 1.19 24.40 1.20 74.40

129
reported literature values. Densities and refractive indices corresponding to
the points on the binodal curve were determined at 24°C and 26°C depend-
ing on the concentration range to avoid turbidity and phase separation. Tie-
line data were determined by analysis of the two layers of a synthetic hetero-
geneous mixture. The mixture was shaken thoroughly and allowed to settle
for at least 5 h in a thermostated settling cell until complete separation was
achieved. The analysis of both layers was made by measuring densities and
refractive indices, and using the standard plots obtained when the binodal
curve was determined. Figure 2 shows one of these standard plots for the
system THF-waterl,1,2-trichloroethane. In the vicinity of the plait point
only density measurements were used, because refractive indices could not
be measured with precision at the plait point composition, as shown in Fig. 2.
TABLE 4
Mutual solubility and tie-line data for THF-water(W)-chloroform(C) at 25°C (values
expressed in weight percent)
Mutual solubility data
THF W C THF W C
0.00 0.08 99.92
16.67 0.41 82.92
29.68 0.86 69.45
40.69 1.81 67.49
53.14 2.38 44.48
62.13 3.38 34.49
66.90 4.25 28.86
71.02 5.44 23.54
73.03 6.16 20.81
74.96 7.36 17.69
76.34 8.94 14.72
77.08 11.21 11.72
76.42 14.68 8.90
75.21 17.05 7.73
72.27 21.66 6.07
69.08 26.89 6.03
65.31 30.48 4.20
62.00 34.51 3.49
66.11 41.44 2.45
60.37 47.92 1.71
44.35 54.51 1.14
38.30 60.94 0.76
32.48 66.91 0.61
26.63 73.10 0.37
20.77 78.89 0.34
14.93 84.69 0.38
9.23 90.23 0.54
3.04 96.19 0.77
0.00 98.81 1.19
Tie-line data
Aqueous phase
THF W C
Organic phase
THF W C
44.30 54.60 1.10 75.20 16.98 7.82
33.50 65.94 0.56 76.50 8.94 14.56
28.80 70.74 0.46 73.61 6.39 20.00
20.77 78.84 0.39 63.60 3.80 32.60
14.90 84.70 0.40 63.10 2.33 44.57
10.10 89.40 0.50 41.50 1.73 56.77
4.00 96.06 0.95 22.37 0.45 77.18 -
 6
6
 7
7
 8
8
 9
9
 10
10
 11
11
 12
12
1
/
12
100%