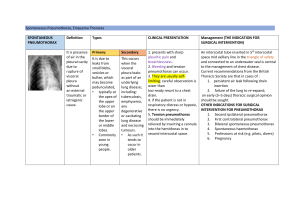
Pediatric Pneumonia: Diagnosis, Investigation & Treatment Guide
Telechargé par
tyamoune

Paediatric pneumonia: a
guide to diagnosis,
investigation and
treatment
Ellen Crame
Michael D Shields
Patrick McCrossan
Abstract
Community-acquired pneumonia remains the leading cause of death
in children under 5 years of age throughout the world. Community ac-
quired pneumonia can include bacterial, viral and fungal causes but it
is very difficult to clinically differentiate between them. Whilst viral
pathogens have been identified as the most common aetiology, bac-
terial infections are considered the more likely to cause severe dis-
ease. It is important that the clinician has the ability to safely
differentiate between those that may require further treatment or
admission to hospital and those that can be managed at home. With
a wide range of presenting symptoms and potential complications,
pneumonia poses a challenge for paediatricians. This article aims to
guide physicians in the management, diagnosis and follow up of chil-
dren with suspected pneumonia, as well as discuss future develop-
ments in this field.
Keywords chest infection; childhood; lower respiratory tract infec-
tion; paediatric; pneumonia
Introduction
Community-acquired pneumonia remains the leading cause of
death in children between the ages of 28 days and 5 years.
1
Pneumonia affects children globally, although is most prevalent
with highest mortality in sub-Saharan Africa and lower socio-
economic regions where vaccines are less accessible.
2
Vaccina-
tion is a cost-effective strategy in preventing death from pneu-
monia
2
and the introduction of the primary childhood vaccine
program in the United Kingdom (UK) significantly reduced the
incidence of pneumonia.
3
Pneumonia has a marked seasonal pattern, with a much
higher prevalence throughout the winter due to the preponder-
ance of infections such as respiratory syncytial virus (RSV),
influenza and pneumococcus.
1
With so many children affected,
pneumonia presents a significant risk to child health and a
burden on healthcare resources. With a wide range of presenting
symptoms and potential complications, pneumonia poses a
challenge for paediatricians. This article aims to guide physicians
in the management, diagnosis and follow up of children with
suspected pneumonia, as well as discuss future developments in
this field.
What is pneumonia?
Currently, no single definition of pneumonia exists that is uni-
versally accepted. Pneumonia is essentially an infection in the
lower respiratory tract (bronchi to alveoli) in which the inflam-
matory process leads to accumulation of fluid in the airspaces
which interferes with gas exchange, leading to the typical
symptoms of tachypnoea, increased work of breathing, hypoxia
and cough.
Traditional pathological studies reported that there are four
pathological stages of pneumonia:
1. Congestion: Over the first 24 hours there is alveolar oedema
and vascular congestion.
2. Red hepatisation: In days 2e4, exudate, containing red blood
cells, neutrophils and fibrin fill the airspaces making them
more solid.
3. Grey hepatisation: In days 5e7, the red cells in the exudate are
beginning to break down.
4. Resolution: From day 8 up to 3 weeks the exudate is broken
down by enzymes, digested by macrophages or coughed up as
sputum.
Pneumonia can be classified based on the radiographic
appearance although this does not necessarily correlate with
severity or aetiology of the disease. Bronchopneumonia (see
Figure 1) appears as bilateral multifocal consolidative patches.
This can progress to lobar pneumonia (see Figure 2) where there
is a large and continuous consolidation of an entire lobe within
the lung. Round pneumonia (see Figure 3) is a phenomenon of
children whereby the radiograph depicts a round, well-
circumscribed opacity most commonly in the lower lobes.
Round pneumonia is thought to be due to underdevelopment of
the pores of Kohn and canals of Lambert, which in adults, allows
dissemination of infection throughout a lobe.
What causes pneumonia?
Community-acquired pneumonia can have bacterial, viral and
fungal causes (see Table 1). Viral pathogens have been identified
as the most common aetiology.
4
These include Respiratory
Syncytial Virus (RSV), Rhinovirus, Influenza, Parainfluenza,
Human Metapneumovirus (hMPV) and Adenovirus. Cytomega-
lovirus (CMV) related pneumonia also needs to be considered in
immunosuppressed patients, particularly those with underlying
HIV infection.
Although viral infections are the more common cause, bac-
terial infections are considered the more likely to cause severe
disease and are responsible for up to 64% of pneumonia-related
deaths.
5
Globally, Streptococcus pnuemoniae and Haemophilus
Ellen Crame MB ChB MRCPCH Paediatric Registrar, Royal Belfast
Hospital for Sick Children (Belfast HSC Trust), UK. Conflicts of
interest: none declared.
Michael D Shields MB ChB MD FRCPCH Professor of Child Health,
Queen’s University Belfast and Consultant Paediatrician, Royal
Belfast Hospital for Sick Children (Belfast HSC Trust), UK. Conflicts
of interest: none declared.
Patrick McCrossan MB BCh BAO MRCPCH MD MSc Academic Clinical
Lecturer, Queen’s University Belfast and Paediatric Registrar, Royal
Belfast Hospital for Sick Children (Belfast HSC Trust), UK. Conflicts
of interest: none declared.
PERSONAL PRACTICE
PAEDIATRICS AND CHILD HEALTH 31:6 250 Ó2021 Elsevier Ltd. All rights reserved.

influenzae remain the most common pathogens. In areas with
good pneumococcal conjugate (PCV) and H. influeznae B (Hib)
vaccine coverage, Staphylococcus aureus and H. influenzae non-B
type are the most prolific. Atypical pneumonias such as Myco-
plasma and Chlamydia account for 27e36% of children admitted
with bacterial pneumonia admitted to hospital
5
(Atypical pneu-
monia is caused by organisms which are difficult to detect on
traditional gram stain and culture methods). This may be due to
the higher rates of hospital admission in the atypical subtypes
due to failure of first line antibiotics. In areas where there are
higher rates of tuberculosis (TB), or travel and immigrations
from these areas, TB needs to be considered as a potential cause.
Fungal causes are particularly important to keep in mind when
assessing immunosuppressed patients, Pneumocystis (PCP)
being the most common cause in HIV infected children.
How does pneumonia in children present?
The symptoms of pneumonia are often nonspecific and can be
very wide ranging, making it a potentially challenging condition
to diagnose. Symptoms also vary with age, although the most
accepted common presenting features of pneumonia in all age
groups include fever, cough, rhinorrhoea, dyspnoea, malaise and
lethargy. Symptoms in infants can include reduced feeding,
grunting breathing and apnoea.
1
Conversely, in older children
more common symptoms are breathlessness, pleuritic chest pain,
abdominal pain and headache.
Clinical examination signs include decreased breath sounds
and focal crepitations on auscultation. These are very sensitive
markers of disease but have a low specificity and are at times
subjective depending on the examiner. Tachypnoea and hypo-
xaemia also have high sensitivity for predicting the presence of a
pneumonia in children of all ages and respiratory rate in partic-
ular can be a very valuable sign in guiding diagnosis and raising
suspicion of disease.
3
Conversely, children with wheeze and low-
grade fever typically do not have pneumonia.
3
Where the diag-
nosis of pneumonia is being considered it is very important to
carefully percuss the chest. Whilst a dull note alone will be often
heard (or felt) in a child with a localised pneumonia, a stony dull
note should alert the clinician to the possibility of a pleural
effusion or empyema.
As seen in Table 1, pneumonia can be caused by bacteria,
fungus and viruses and it is very difficult to clinically differen-
tiate between the underlying causes. However, a higher index of
suspicion for bacterial infection should be considered in children
presenting with persistent fever, breathlessness and increased
work of breathing in the absence of wheeze.
3
Investigations
There are no investigations recommended for children with
pneumonia who can be safely managed in the community. For
those requiring secondary care assessment and admission to
hospital, the physician may consider the following:
Microbiological tests
Sputum gram stain and culture is a useful test to determine the
underlying bacterial causative organism. However, sputum
samples are notoriously difficult to obtain in younger children
and take several days to culture. They are particularly useful for
the child who is severely unwell or not responding to treatment,
in order to target antimicrobial therapy. Also, if admitted to the
Intensive Care Unit, a bronchoalveolar lavage (BAL) sample is
readily attainable via their endotracheal tube.
Nasopharyngeal aspirates (NPA) can be sent for viral poly-
merase chain reaction (PCR). NPA are sensitive for detecting the
presence of viruses but are not adequate for determining bacte-
rial causes as the presence of normal nasal bacterial flora affects
the interpretation.
Rapid detection of the capsular polysaccharide (CPS) antigen
of Streptococcus pneumoniae from urine samples has shown
some promise with a high sensitivity and negative predictive
value for detecting pneumococcal infection. However, its low
specificity undermines its clinical utility.
6
Imaging
The British Thoracic Society (BTS) guidelines state that,
‘children with symptoms and signs suggesting pneumonia
who are not admitted to hospital should not routinely have a
chest X-ray’.
3
Chest radiographs should not be considered a
routine investigation for children with suspected pneumonia,
nor is it necessary to make the diagnosis. Reviewing the
literature on studies of chest x-rays, there can be no
Figure 1 Bronchopneumonia.
Figure 2 Lobar pneumonia.
PERSONAL PRACTICE
PAEDIATRICS AND CHILD HEALTH 31:6 251 Ó2021 Elsevier Ltd. All rights reserved.

significant links made between radiological findings and
aetiology.
3
The finding on chest x-rays also play no signifi-
cant role in guiding the management of the pneumonia when
the decision whether to treat with antibiotics is based on
clinical findings and the response of symptoms to treatment.
7
In addition, chest x-rays can be challenging to interpret, and
findings are often subjective. The interpretation of x-rays is
limited by the quality of the film and dependant on the
expertise of the reader.
8
Many studies have demonstrated
significant variability and discrepancy between chest X-ray
findings when interpreted by both radiologists and clinicians.
8
However, chest x-rays do play a role when complications of
pneumonia are suspected. Chest X-ray should be considered
where symptoms are persistent and where there is an inad-
equate response to treatment within 72 hours.
8
Blood tests
Collating the data from multiple studies, the BTS concluded that,
‘acute phase reactants are not of clinical utility in distinguishing
viral from bacterial infections and should not routinely be
tested’.
5
Both viral and bacterial infections can cause a rise in C
Reactive Protein (CRP) and as a result the CRP should not in-
fluence the decision to treat for bacterial illness. However, CRP
does play a role when complications are suspected, and a rising
CRP may be an indication for more complex disease not
improving with existing treatment. Neither a raised white cell
count (WCC) nor erythrocyte sedimentation rate (ESR) has been
proven to differentiate between viral and bacterial aetiologies.
9
Procalcitonin (PCT) is released as part of the proinflammatory
response of the innate immune system. It reaches detectable
leaves at a faster rate than other inflammatory markers such as
CRP and is not raised by viruses or collagen vascular diseases. A
study by Baumann et al. suggests the elevated PCT is a good
marker for pneumococcal infection and as a result, elevated PCT
could be used to guide the treatment with antibiotics in children
with a suspected diagnosis of pneumonia.
10
Management
The first step in managing children with pneumonia is deciding
whether or not they can be managed safely in the community, or
whether they need referring to a hospital. A thorough assessment
of disease severity is needed on first presentation, with the
premise that previously well children with mild disease are best
managed at home. An assessment of severity will also influence
the decision to investigate and initiate treatment, as well as guide
the duration of treatment and level of medical and nursing care
required in the hospital setting.
The initial assessment of a child presenting with infective
symptoms will normally take place in the primary care or
emergency department setting. Doctors will assess children
based on their clinical presentation but will also need to consider
associated risk factors. Underlying health conditions and the
social background of the child can impact on the ability for the
condition to be well managed in the community. Children with
complex needs and chronic underlying lung conditions may be
more vulnerable to severe disease and may have a lower respi-
ratory reserve. As a result, there is a lower threshold for initiation
of antibiotic treatment and admission to hospital.
Unlike the CURB 65 score in adults, there is no reliable
assessment tool for scoring severity of disease in children.
3
As a
result, clinical markers of severity (see Table 2) remain the gold
standard for assessing children that need hospital care. One of
the most important measures to guide the need for admission to
hospital is oxygen saturations, with hypoxaemia being a sensi-
tive indicator of disease severity and prognosis.
11
Also, tachyp-
noea correlates with hypoxaemia and so the respiratory rate
requires careful assessment.
The severely unwell child may require admission to the Pae-
diatric Intensive Care Unit (PICU). The two main scenarios where
PICU is indicated are when the pneumonia is severe enough to
cause respiratory failure, requiring ventilatory support, or when
the pneumonia is complicated by septicaemia. The diagnosis of
respiratory failure is made following blood gas analysis. In chil-
dren outside of the PICU setting, capillary or venous blood gases
are most commonly used as arterial sampling is painful and very
challenging in smaller children who do not have an arterial
catheter. Respiratory failure would be indicated by low PaO
2
readings and a high PaCO
2
which may indicate the need for
respiratory support and thus referral to a high dependency unit.
Septicaemia may present as tachycardia, tachypnoea, hypo-
xaemia, signs of shock or recurrent apnoea with irregular
breathing patterns.
Treatment
For children that do not warrant hospital admission, robust
safety netting advice should be implemented to ensure that
parents are aware of the signs of worsening disease, as discussed
above.
The mainstay of hospital treatment is supportive manage-
ment. This may involve oxygen therapy if pulse oximetry reveals
oxygen saturations lower than 92%.
3
Intravenous fluids may be
indicated when the child is struggling to maintain oral input due
to breathlessness and fatigue, to prevent dehydration. Nasogas-
tric tubes, although useful for providing hydration in many sce-
narios, should be avoided where possible in smaller children as
Figure 3 Round pneumonia.
20
Source: reproduced from reference 20
with permission of Springer-Verlag 2010.
PERSONAL PRACTICE
PAEDIATRICS AND CHILD HEALTH 31:6 252 Ó2021 Elsevier Ltd. All rights reserved.

they can cause further breathing compromise by obstructing the
smaller nasal passages.
3
With the use of intravenous fluids
comes the risk of electrolyte imbalance, made more pronounced
in pneumonia by the potential development of the syndrome of
inappropriate anti-diuretic hormone (SIADH). Therefore, elec-
trolyte and sodium monitoring are required to prevent hypona-
traemia in instances where prolonged intravenous fluids are
administered.
Chest physiotherapy is not felt to be beneficial in children
with uncomplicated pneumonia. Chest physiotherapy may
potentially prolong fever duration and exacerbate breathing dif-
ficulties
12
and is not recommended as part of management for
children with pneumonia by the British Thoracic Society. Chest
physiotherapy, along with the use of mucolytic agents, continues
to play a role in airway clearance in some children with complex
needs who may struggle to independently clear secretions. In
addition, where acute airway collapse is secondary to mucoid
secretions causing plugging in the bronchi, assisted airway
clearance will again be an important part of the management
strategy.
The world health organisation (WHO) defines pneumonia as,
‘the presence of either fast breathing or lower chest wall in-
drawing where the child’s chest moves in or retracts during
inhalation.
1
’ The WHO states that due to the high mortality and
morbidity rates worldwide, a child with suspected pneumonia
should be treated with antibiotic therapy. This WHO definition of
pneumonia was developed in order to be easily implemented by
primary health care workers in the developing world to guide their
use of prescribing antibiotics and thus prevent the serious
sequelae of what is the most common cause of serious illness and
death in young children worldwide. This was successful and
resulted in a significant reduction in all-cause mortality for chil-
dren. However, such high sensitivity comes at the cost of speci-
ficity, failing to distinguish between bacterial and viral causes.
Indeed, research into use of the WHO guidelines in low-income
countries has identified overdiagnosis of pneumonia in cases of
wheezing, with consequent underdiagnosis of asthma, leading to
significant respiratory morbidity and, perhaps, even mortality.
13
As previously discussed, viral aetiology is more frequent than
bacterial and there is no evidence to support that either chest X-
ray findings or inflammatory markers can reliably distinguish
between the two. There has been much consideration given to
the extensive use of antibiotics and the economic and health
burden that results.
14
BTS guidelines state that in a child under
two years with mild symptoms, the cause is most likely to be
viral in nature.
3
Where the child appears acutely unwell, im-
mediate administration of antibiotics is recommended, however
it may be reasonable to carefully consider the use of antibiotics in
mild disease.
14
If deciding upon prescribing antibiotics, Amoxicillin is recom-
mended as first line treatment of lower respiratory tract infection,
as it has effective coverage against the most common pathogens in
children.
3
BTS guidance states that oral amoxicillin should be used
in the first instance though there may be local microbiological
guidelines in place depending on the prevalence of antibiotic
Common pathogens causing pneumonia in children
Age Bacteria Viruses
<20 days E. coli, Group B Streptococcus,Listeria Herpes simplex virus, Cytomegalovirus
3 weekse3 months Chlamydia trachomatis,Streptococcus
pneumoniae,Pertussis,Haemophilus
influenzae,Staphylococcus aureus,Moraxella
catarrhalis
Adenovirus, respiratory syncytial virus,
parainfluenza, influenza
4 monthse5 years Streptococcus pneumoniae,Mycoplasma,
Haemophilus influenzae,Staphylococcus
aureus
Adenovirus, rhinovirus, influenza,
parainfluenza, varicella zoster, respiratory
syncytial virus
>5 years Streptococcus pneumoniae,Mycoplasma,
Staphylococcus aureus,Tuberculosis
Adenovirus, Epstein Barr virus, influenza,
rhinovirus
Table 1
Clinical indicators of severity in childhood pneumonia
5
Mild/moderate Severe
Infants Temp <38.5C
RR <50 breaths/minute
Mild recession
Taking full feeds
Temp >38.5C
RR >70 breaths/minute
Moderate/severe recession
Cyanosis
Intermittent apnoea
Poor feeding
Capillary refill time
>2 second
Tachycardia
Older children Temp <38.5C
RR <50 breaths/minute
Mild breathlessness
No vomiting
Temp >38.5C
RR >50 breaths/minute
Severe difficulty in
breathing
Nasal flaring
Grunting
Cyanosis
Signs of dehydration
Tachycardia
Capillary refill time
>2 seconds
Table 2
PERSONAL PRACTICE
PAEDIATRICS AND CHILD HEALTH 31:6 253 Ó2021 Elsevier Ltd. All rights reserved.

resistant pathogens. Enteral administration of antibiotics is as
effective as intravenous. This reduces the need for cannulation
and can be continued in the community effectively.
3
Intravenous
antibiotics may be required where complications are suspected or
when the enteral route is poorly tolerated.
3
Macrolide antibiotics
(e.g. azithromycin, clarithromycin) are second line treatment if
there is an unsatisfactory response to amoxicillin or in instances
where an atypical pneumonia is suspected. Co-amoxiclav may be
a better first line antibiotic in children with complex needs asso-
ciated with a poor cough and swallowing dysfunction for whom
anaerobic bacteria may be easily aspirated.
Figure 4 illustrates a suggested stepwise approach to the
diagnosis and management of children with suspected
pneumonia.
Complications
Most children with community-acquired pneumonia go on to
make a full recovery. However, both pulmonary and systemic
complications occur in around 3%,
5
resulting in significant
morbidity and mortality. Regular reassessment of a child with
pneumonia is recommended, including for children managed in
the community. Increased effort of breathing, agitation and
persistent or swinging fevers should prompt parents to return for
further assessment and it is important that this information is
provided. Pulmonary complications include parapneumonic
effusion, empyema and lung abscess. Systemic complications can
include multi organ failure, metastatic infection, bacteraemia and
Acute Respiratory Distress Syndrome (ARDS).
Empyema and pleural effusion
A pleural effusion is a collection of fluid in the space between the
lungs and the chest wall (the pleural space) (see Figure 5). When
this pleural fluid is pus or infective then it is termed an empyema.
They occur in 1% of cases of community-acquired pneumonia.
However, in patients needing hospital admission this may be up
to 40%.
5
Empyema should be suspected if fevers are persisting
on adequate treatment for 48 hours, or there has been 7 days of
persistent fever. The investigation of choice is a chest X-ray to
identify fluid in the pleural space and ultrasound to estimate the
volume of the fluid present.
Intravenous antibiotic therapy is necessary in these patients to
provide a broader coverage and higher penetrance of the pleural
space.
3
Local microbiology advice should be sought to guide the
antimicrobial treatment but should ensure cover for S. pneumo-
niae. These children often require a prolonged course of enteral
antibiotics (1e4 weeks), even once the intravenous antibiotics
have been discontinued.
Effusions which compromise respiratory function should not be
managed by antibiotics alone and early insertion of a chest drain
should be considered. Chest drains should be placed under ultra-
sound guidance and a small bore (including pig tail) drain should be
considered ahead of large bore surgical drains where possible.
Intrapleural fibrinolytics shorten hospital stay and are rec-
ommended for any complicated parapneumonic effusion. Pa-
tients should be considered for surgical treatment (including
video assisted thoracoscopy (VATS)) if they have persistent
sepsis in association with a pleural collection despite chest tube
drainage and antibiotics.
15
Necrotising pneumonia and lung abscess
Lung abscess is a collection of pus within the lung tissue and is
considered a very rare but significant complication in children,
due to its high rates of mortality and long-term implications. This
occurs when there is liquefactive necrosis (in which the lung
tissue is digested by hydrolytic enzymes resulting in a circum-
scribed lesion containing pus) of lung tissue (secondary to
infection) causing the formation of a cavity containing inflam-
matory cells, bacteria and frank pus.
Lung abscess may be identified on chest X-ray by the presence
of a cavity with an air-fluid level. A bronchopleural fistula may
form, leading the abscess into the pleural space with subsequent
formation of an empyema or pneumothorax.
Treatment includes a prolonged course of intravenous anti-
biotics. Percutaneous CT guided drainage of the abscess may be
required and in cases where there is progressive lung paren-
chymal necrosis, segmental or lobar resection may be necessary.
Long term complications of these conditions can occur
causing bronchiectasis, scarring and chronic cough.
16
Pneumatocoele
Pneumaotocoeles are intrapulmonary air-filled cysts. As a
consequence of pneumonia, the bronchus may become narrowed
by inflammatory exudates, leading to the formation of a ball
valve that causes distal dilatation of the alveoli as air is able to
enter the cystic space but not leave it.
Pneuomatocoeles appear as thin-walled cystic spaces con-
taining air (see Figure 6). If they are imaged during the early
stage of the infection, they may have surrounding consolidation
which makes it difficult to distinguish from an abscess.
The main significance of pneumatocele is that it must be distin-
guished from other cavitary pulmonary lesions such as an abscess in
order to avoid unnecessary invasive interventions. Most pneuma-
tocoeles spontaneously resolve over time (usually by six weeks)
following appropriate treatment of the underlying infection.
Decompression is considered when the pneumatocoele is large
enough to compress adjacent lung and mediastinal structures.
Follow up
BTS guidelines do not recommend routine follow up imaging in
uncomplicated community-acquired pneumonia. If complica-
tions arise such as empyema or persistent lobar collapse, then
this may warrant follow up X-ray.
5
Interestingly, children with a
round pneumonia who respond to antibiotics do not require
follow up radiograph (unlike the management of this phenome-
non in adults).
17
Future developments
Bedside ultrasound
Ultrasound is diagnostic tool currently used in many paediatric
intensive care settings and could reduce the need for radiation
exposure and chest x-rays in the future. The benefits of ultra-
sound include the absence of radiation and also high
PERSONAL PRACTICE
PAEDIATRICS AND CHILD HEALTH 31:6 254 Ó2021 Elsevier Ltd. All rights reserved.
 6
6
 7
7
 8
8
1
/
8
100%