Autocrine Growth of Transitional Cell Carcinoma of the Bladder Induced by Granulocyte-Colony

(CANCER RESEARCH 55, 3438-3443. August I. 1995]
Autocrine Growth of Transitional Cell Carcinoma of the Bladder Induced by
Granulocyte-Colony Stimulating Factor1
Masaaki Tachibana,2 Ayako Miyakawa, Hiroshi Tazaki, Kayoko Nakamura, Atsushi Kubo, Jun-ichi Hata,
Tatsunari Nishi, and Yasuhiro Amano
Departments of Urology ¡M.T.. A. M., H. T.], Radiology [K. N., A. K.I, and Pathology fj-i. ti.}. School of Medicine, Keio University, 35-Shinanomachi. Shinjukit-ku, Tokyo,
Japan, and Tokyo Research Laboratories, Kyowa Hakko Kogyo Co., Liti., Tokyo, Japan [T. N., Y. A.]
ABSTRACT
Granulocyte-colony stimulating factor (G-CSF) produced by nonhema-
topoietic malignant cells has been reported to be capable of inducing a
leukemoid reaction in the host through intense stimulation of leukocyte
production. Furthermore, this is frequently associated with aggressive
tumor cell growth and a detrimental clinical outcome. In this study, we
identified bladder cancer cells producing G-CSF with the expression of
the functional receptor, which provides direct evidence of autocrine
growth of bladder cancer cells induced by G-CSF. The cancer cells used
in this study were obtained from a 76-year-old man who had a metastatic
transitional cell carcinoma of the bladder and who demonstrated marked
leukocytosis; his peripheral blood leukocyte count was 94,900 leukocytes/
mm3, and his serum G-CSF level was 103 pg/ml. The culture medium in
which the cancer cells were grown exclusively contained a significant
amount of G-CSF (5560 pg/ml). Significant G-CSF inKN A expression and
G-CSF receptor mRNA expression in the cultured cells were demon
strated by the reverse transcription-PCR method. In addition, binding
studies with the use of radiolabeled recombinant G-CSF demonstrated the
presence of high-affinity G-CSF binding receptors on the cultured cancer
cells. Finally, the proliferation of the cultured cancer cells was stimulated
by exogenous G-CSF administration, and this stimulation was inhibited by
adding anti-G-CSF antibody, as demonstrated by both the flow cytometric
bromodeoxyuridine incorporation technique and the | 'lI |lh\miilinc incor
poration assay. These results strongly suggest that G-CSF production by
the bladder cancer cells studied augments autocrine growth. Therefore,
we recommend exercising caution in the clinical use of G-CSF for bladder
cancer patients.
INTRODUCTION
G-CSF3 produced by nonhematopoietic malignant cells has been
reported to be capable of inducing a leukemoid reaction in the host
through intense stimulation of leukocyte production (1-11). This is
frequently associated with aggressive tumor cell growth and a detri
mental clinical outcome (8-12).
Varieties of nonhematopoietic malignant tumors, including bladder
carcinoma (2-4), hepatoma (5), mesothelioma (6), squamous cell
carcinoma of the oropharynx (7), melanoma (8), glioblastoma (9), and
sarcoma (10, 11), have been demonstrated to secrete G-CSF in
amounts large enough to cause a significant systemic hematopoietic
effect. In addition, receptors for G-CSF have also been confirmed on
the cell surfaces of several nonhematopoietic cell types, including
human placenta and trophoblastic cells (13), human vascular endo-
thelial cells (14), and cell lines derived from human small cell carci
noma of the lung (15). Previously, we reported the expression of
Received 2/10/95; accepted 5/30/95.
The costs of publication of this article were defrayed in part by the payment of page
charges. This article must therefore he hereby marked advertisement in accordance with
18 U.S.C. Section 1734 solely to indicate this fact.
1This work was supported in part by Grants-in-Aid 04404064 and 04454409 for
Scientific Research from the Ministry of Education, Science and Culture. Japan.
2 To whom requests for reprints should be addressed.
'The abbreviations used are: G-CSF, granulocyte-colony-stimulating factor; GM-
CSF. granulocyte-macrophage colony-stimulating factor; RT-PCR, reverse transcription-
PCR; BrdUrd, bromodeoxyuridine; LI, labeling index; SCID, severe combined immuno
deficiency; CHO, Chinese hamster ovary.
functional G-CSF receptors in transitional cell carcinoma of the
bladder (16).
The above observations lead naturally to the tempting speculation
that simultaneous acquisition of the ligand (G-CSF) production and its
receptor expression by a malignant tumor may provide a strong
autocrine growth advantage. This study addresses our recent obser
vations, which strongly suggest that such autocrine growth promotion
of malignant tumor cells by G-CSF does, in fact, take place.
MATERIALS AND METHODS
The cancer cells used in this study were obtained from a 76-year-old man
with metastatic transitional cell carcinoma of the bladder who demonstrated
marked leukocytosis; his peripheral blood leukocyte count was 94.900 leuko-
cytes/mttr', and his serum G-CSF level was 103 pg/ml. Immunohistochemical
study of the cancer tissue obtained by biopsy with the use of a mAb against
recombinant human G-CSF was performed to identify the exact cell type
responsible for G-CSF production. Sections of the 5% formalin-fixed and
paraffin-embedded bladder tumor tissue specimen were studied by using the
avidin-biotin-peroxidase method. Mouse anti-human G-CSF mAb (KW341)
provided by Kyowa Hakkou Co, Ltd. (Tokyo, Japan) was used as the primary
antibody at a dilution of 1:50. To confirm the specificity of the immunohis-
tochemical study, tumor specimens from SCID mice implanted with CHO cells
transfected with human G-CSF cDNA (17) were examined as a positive
control, and as a negative control, mouse IgG was used as the primary antibody
instead of anti-G-CSF antibody.
The tumor tissue was minced into 1-mm3 pieces, placed on a culture flask
(25 cm2), and maintained in culture medium [RPMI 1640 and Eagle's MEM
diluted 1:1, supplemented with 10% heat-inactivated PCS, 1% insulin-trans-
ferine-sodium selenite medium supplement (Sigma, Japan), 100 (xg/ml strep
tomycin, and 100 international units/ml penicillin] in an atmosphere of 5%
CO, for additional culture.
Exponential growth of the cells was seen approximately 3 weeks after the
primary culture. Subsequently, the cells were subcultured with a split ratio of
1:10 every 4-6 days. G-CSF concentrations in the culture medium, on which
the cancer cells were grown exclusively, were measured by ELISA.
Both G-CSF and G-CSF receptor mRNA expression on the cultured cancer
cells were studied with the use of the RT-PCR method. Total RNA samples
were purified from the cultured cancer cells by the acid guanidine phenol-
chloroform method (18). The respective RNA (5 /ig) samples were converted
into cDNA with the use of oligo(dT) primers and reverse transcriptase (code
8089SA; Life Technologies) diluted with H2O to obtain 100 fj.1of the cDNA
preparation. Five-fil samples were subjected to the following PCR: («)the
ß-actin-specific fragment was detected by PCR (20 cycles at 94°Cfor 1 min,
65°C for 1 min, and 72°C for 3 min) with primers 5'-GATATCGC-
CGCGTCGTCGTCGAC-3' (forward primer) and 5'-CAGGAAGGAAG-
GCTGGAAGAGTGC-3' (reverse primer); (b) the G-CSF-specific 278-bp
fragment was detected by PCR (40 cycles at 94°Cfor 1 min, and 50°Cfor 1
min) with 5'-CTGTGTGCCACCTACAAG-3' (forward primer) and 5'-GC-
CATTCCCAGTTCTTCC-3' (reverse primer); and (c) the G-CSF receptor
a-chain 727-bp fragment was detected by PCR (35 cycles at 94°Cfor 1 min,
65°Cfor 1 min, and 72°Cfor 1 min) with 5'-ACAGTCCTCACCCTGAT-
GACCT-3' (forward primer) and 5'-TGCCTCTTAAAGGCCTGAGCTA-3'
(reverse primer). In addition, as markers of other hematopoietic growth factors,
GM-CSF and GM-CSF receptor mRNA expression on the cultured cancer cells
were studied by RT-PCR; (d) the 441-bp GM-CSF-specific fragment was
detected by PCR (43 cycles at 94°Cfor 30 s, and 63°Cfor 1 min) with primers
5'-CTGGAGATGTGGCTGCAGAGCC-3' (forward primer) and 5'-TGCT-
3438
on July 8, 2017. © 1995 American Association for Cancer Research. cancerres.aacrjournals.org Downloaded from

AUTOCRINE CELL GROWTH WITH G-CSF
GGGAGCCAGTCCAGGAGTGA-3' (reverse primer); and (e) the 621-bp
GM-CSF receptor «-chainfragment was detected by PCR (40 cycles at 94°C
for 1 min, 60°Cfor 1 min, and 72°Cfor 1 min) with primers 5'-TGACCAG-
CACCATGCITCTCCT-3' (forward primer) and 5'-ACCACCCGAGAAAT-
TGGCATCCAA-3' (reverse primer). To further confirm that the amplified
products originated from the respective cDNA, they were subjected to appro
priate restriction enzyme digestions. In addition, each RT-PCR was performed
without the reverse transcriptase reaction as a negative control.
Stimulation of cultured cancer cells by exogenous G-CSF administration
and neutralization of its growth-promoting activity by anti-G-CSF antibody
under serum-free conditions were studied. The proliferating activity of the
cultured cancer cells was measured by the flow cytometric BrdUrd incorpo
ration technique. The cells were incubated in 1 ml of a 1:1 mixture of RPMI
1640 and Eagle's MEM without serum supplementation in 12-well culture
dishes (well diameter, 22 mm; Corning) at 37°Cin a humidified atmosphere of
5% CO2 with 95% air. Serial concentrations of recombinant mutant human
G-CSF, kindly provided by Kyowa Hakko Kogyo Co., Ltd. (KW-2228; 3, 7),
were added every 24 h for a total of three times. Twenty-four h after the final
G-CSF treatment, BrdUrd was added to each culture well at a final concen
tration of 5 /xg/ml, and incubation was continued for another hour. The cells
were harvested with 0.25% trypsin and 1 mM EDTA and were then washed
twice. The cells were subsequently stained with FITC-labeled anti-BrdUrd
antibody and then poststained with 0.5% propidium iodide. The double-stained
cells were analyzed with an Epics ELITE flow cytometer (Coulter, Hialeah,
FL), and the LI, i.e., the number of cells stained with BrdUrd divided by the
total estimated cell count, was calculated.
For the neutralizing test, 0.5 /ig/ml concentrations of G-CSF were prein-
cubated with or without serial concentrations of anti-human G-CSF antibody
(IgG class; R&D Systems, Minneapolis, MN) before their addition to the
cultured cells. The experiment was otherwise carried out in exactly the same
way as the stimulation test. It was also demonstrated whether the presence of
a specific anti-human G-CSF antibody would inhibit tumor cell proliferation.
The antibody was added every 24 h for a total of three times to cell cultures
with or without serial concentrations of anti-human G-CSF antibody (R&D
Systems) under a scrum-free condition. The experiment otherwise was carried
out in exactly the same way as the neutralizing test. KU-7 cells (19) derived
from human bladder cancer and not exhibiting functional G-CSF receptors
were used as control cells.
Exactly the same experiments were carried out by the [%H]thymidine incor
poration method. The bladder carcinoma cells (1 X 10J) were incubated in 0.1
ml of the culture medium without PCS in a 96-well microtitcr tray (Nunc,
Roskilde, Denmark). Twenty-four h after the final G-CSF and/or anti-G-CSF
antibody treatments, DNA synthesis in the cultures was determined by addi
tional [methyl-3H]thymidine (Amersham, Amersham, UK) (0.6 fiCi/well; 1
Ci = 37 MBq) during a 4-h pulse. Cells were harvested onto glass fiber filters
and counted with a liquid scintillation counter (I.S. 9800; Beckman Instru
ments Inc., Fullerton, CA).
G-CSF receptor binding experiments were conducted with the use of the
following method. Na'25I (DuPont-NEN) and Enzymobead reagent (Bio-Rad)
were used. Recombinant mutant G-CSF (KW-2228) served as the ligand. The
KW-2228 was radioiodinated with 37 MBq of Na125I with the use of the
solid-phase glucose oxidase-lactoperoxidase method, as described by Piao and
Okabe (20). The specific activity of radioiodinated KW-2228 was 6 X IO6
cpm/fig protein. The cultured cells were incubated for 24 h at 4°Cin 24-well
tissue culture plates in 0.5 ml of isotonic PBS containing 0.2% BSA and with
or without 125I-labeled KW-2228. After incubation for 24 h, the medium was
aspirated and the cells were washed with cold PBS. The cells were then
solubilized in 0.25 ml of 2 M NaOH, and the radioactivities were measured.
Nonspecific binding (binding of I25l-Iabeled KW-2228 to the cells in the presence
of G-CSF at 1000 ng/0.5 ml), which ranged between 6-18%, was subtracted from
the total binding to determine the specific binding. The specific binding of the
labeled KW-2228 was expressed as the percentage of binding measured in the ab
sence of unlabeled KW-2228. The Scatchard plot analysis of the specific binding
of 125I-labeled KW-2228 to the cells was estimated.
RESULTS
The cancer cells used in this study were obtained from a 76-year-
old man with metastatic transitional cell carcinoma of the bladder. The
patient underwent radical cystectomy for invasive carcinoma of the
bladder on June 28, 1993. Pathological analysis of the excised bladder
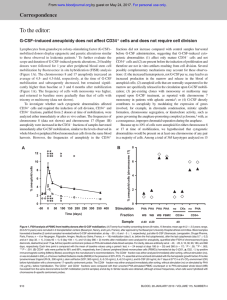
Fig. 1. Immunohistochemical staining with the use of anti-G-CSF mAb. Sections of the 5% formalin-fixed and paraffin-embedded bladder tumor tissue specimen were studied hy
the avidin-biotin-peroxidase method. The mAb that reacted with the tumor cells is shown as brown-colored granular staining, primarily involving the cytoplasm (A; X 400). The CHO
cells transfected with human G-CSF cDNA and transplanted in SCID mice as a positive control were strongly positively stained (B; X 400). 0, negative control staining.
3439
on July 8, 2017. © 1995 American Association for Cancer Research. cancerres.aacrjournals.org Downloaded from

AUTOCRINE CELL GROWTH WITH G-CSF
=•6000r
Day 0 Day 2 Day5 Day?
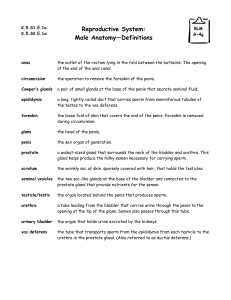
Fig. 2. G-CSF concentrations in the culture media as measured by the ELISA method.
The culture media, on which the cancer cells grew exclusively, contained a significant
amount of G-CSF, and the increase in the number of cancer cells during culturing
paralleled that reached in 5560 pg/ml of medium after 7 days of culture.
demonstrated transitional cell carcinoma, grade-3, pT3b, ly(-), v(+),
pNO, MO. Six months after the procedure, he experienced urethral
bleeding and was diagnosed as having urethral recurrence. He under
went total urethrectomy on December 28, 1993. Subsequently, peri
toneal tumor recurrence and multiple lung métastasesdeveloped,
revealing marked leukocytosis; his peripheral blood leukocyte count
was 94,000/mm3, and his serum G-CSF level was 103 pg/ml.
Immunohistochemical study of the cancer tissue obtained by biopsy
with the use of mAb against recombinant human G-CSF was per
formed to identify the exact cell type responsible for G-CSF produc
tion. The mAb that reacted with the tumor cells is shown as a
brown-colored granular staining, primarily involving the cytoplasm.
The positive staining was limited to the cancer cells and no other cell
types, such as fibroblasts or infiltrating monocytes, were affected (Fig.
L4). The CHO cells transfected with human G-CSF cDNA and
transplanted in SCID mice as a positive control were strongly posi
tively stained (Fig. IB). The culture medium, on which the cancer
cells were grown exclusively, contained a significant amount of
G-CSF, and the increase in the number of the cancer cells during
culture paralleled that reached in the medium containing 5560 pg/ml
after 7 days of culture (Fig. 2).
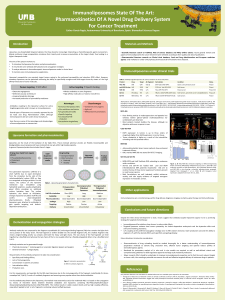
G-CSF and G-CSF receptor mRNA expression on the cultured
cancer cells were studied with the use of RT signals for both G-CSF
and G-CSF receptor and were detected, as shown in Fig. 3, B and C.
The RT-PCR product exhibited a specific G-CSF transcription signal
of 278 bp and a G-CSF receptor signal of 727 bp in samples from the
cultured cells. The RT-PCR product demonstrated a specific GM-CSF
transcription signal of 441 bp (Fig. 3D) but no definitive GM-CSF
receptor transcription signal of 621 bp (£).
Stimulation of cultured cancer cells by exogenous G-CSF admin
istration and neutralization of its growth-promoting activity by anti-
G-CSF antibody were studied by the flow cytometric BrdUrd incor
poration technique. As shown in Fig. 4A, the BrdUrd Lis were 12.7,
14.0, and 16.7% for 0, 0.1, and 0.5 jug/ml G-CSF concentrations,
respectively. Therefore, proliferation of the cultured cancer cells was
stimulated by G-CSF. Meanwhile, when 0.5 /xg/ml G-CSF was pre-
incubated with serial concentrations of anti-G-CSF antibody, BrdUrd
Lis were 17.2,14.3, and 12.7% for 0,10, and 50 /¿g/mlconcentrations
of anti-G-CSF antibody, respectively (Fig. 4B). Therefore, stimulation
of cell proliferation by G-CSF was inhibited by anti-G-CSF antibody.
The addition of 50 ¡¿gof anti-G-CSF antibody neutralized the cell
growth stimulated by 0.5 jag/ml of G-CSF by 26.2%. Furthermore,
when the cells were cultured with anti-G-CSF antibody, BrdUrd Lis
were 11.7, 10.4, 9.5, and 8.9% at anti-G-CSF antibody concentrations
of 0, 10, 50 /j,g, and 200 jug/ml, respectively (Fig. 5).
In addition, [3H]thymidine uptake of the cells was illustrated in
Table 1. The uptake at 24-h incubation after the final G-CSF admin
istration with 0.1 and 0.5 /xg/ml was 5544.7 ± 680.1 and
6030.8 ±409.5 cpm, respectively. These were significantly higher
than those of controls (4760.6 ±309.1 cpm; P < 0.05). In addition,
0.5 /n.g/ml G-CSF was preincubated with serial concentrations of
anti-G-CSF antibody, and [3H]thymidine incorporations were
6180.8 ±285.3, 4849.8 ±216.5, and 4373.0 ±278.1 cpm in 0, 10,
and 50 jug/ml concentrations of anti-G-CSF antibody, respectively.
[3H]thymidine incorporations with anti-G-CSF antibody cultures were
I I
eoibp- 278bp- 441bp-
0-Kt'm G-CSF G-CSF GM-CSF GM-CSF
receptor receptor
ABODE
Fig. 3. Detection of G-CSF mRNA and G-CSF receptor mRNA by the RT-PCR method. A. the ß-actin-specific fragment was detected by PCR (20 cycles at 94°Cfor 1 min, 65°C
for 1 min, and 72°Cfor 3 min) with primers 5'-GATATCGCCGCGTCGTCGTCGAC-3' (forward primer) and 5'-CAGGAAGGAAGGCTGGAAGAGTGC-3' (reverse primer); B. the
G-CSF-specific 278-bp fragment was detected by PCR (40 cycles at 94°Cfor 1 min, and 50°Cfor 1 min) with 5'-CTGTGTGCCACCTACAAG-3' (forward primer) and
5'-GCCATTCCCAGTTCTTCC-3' (reverse primer); C. the G-CSF receptor a-chain 727-bp fragment was detected by PCR (35 cycles at 94°Cfor 1 min, 65°Cfor 1 min, and 72°C
for 1 min) with 5'-ACAGTCCTCACCCTGATGACCT-3' (forward primer) and 5'-TGCCTCTTAAAGGCCTGAGCTA-3' (reverse primer); D, the 441-bp GM-CSF-specific fragment
was detected by the PCR (43 cycles at 94°Cfor 30 s, and 63°Cfor 1 min) with primers 5'-CTGGAGATCTGGCTGCACACCC-3' (forward primer) and 5'-TGCTGGGAGCCACTC-
CAGGAGTGA-3' (reverse primer); and £,the 621-bp GM-CSF receptor a-chain fragment was detected by PCR (40 cycles at 94°Cfor 1 min, 60°Cfor 1 min, and 72°Cfor 1 min)
with primers 5'-TGACCACCACCATGCTTCTCCT-3' (forward) and 5'-ACCAGCCCAGAAATTCGCATCCAA-3' (reverse primer). To further confirm that the amplified products
originated from the respective cDNA, they were subjected to appropriate restriction enzyme digestions. Size markers from lop, 4.3, 1.8, 1.1, 0.68, 0.38, 0.25, and 0.12 kb. RT-PCR
exhibited a 278-bp band signal for G-CSF (B), a 727-bp band signal for the G-CSF receptor (C), and a 441-bp band of GM-CSF (D) in samples from the cultured cells. However,
the 621-bp band of GM-CSF receptor was not identified in the sample from the cultured cells (£).
3440
on July 8, 2017. © 1995 American Association for Cancer Research. cancerres.aacrjournals.org Downloaded from

AUTOCRINE CELL GROWTH WITH G-CSF
o.COto05rra8'Sso.nso
ir»"u
9-ED
eo'S
g-«3
_jliÃ-
O 10 20 30 40 50 60
PI-DNA
Control LI 12.7%
O 10 20 30 40 50 60
PI-DNA
GCSFO.Imcg/mi LI 14.0%
O IO 20 30 40 50 6
PI-DNA
GCSFO. Smog/mi LI 16.7%
B
u
EÃŒBr:i^JL^i,,,,.0
.S-°
5
EI8'OD
CD
CM"0
.
0 -:W;:•:;•;',
/ :
S
IO 20 30 40 50 60
PI-DNA
GCSF 0.5mcg/m/ LI 17.2%
IO 20 30 40 50
PI-DNA
fcg^
60
Anti-GCSF lOmcg/nÃ- LI 14.3%
O IO 20 30 40 50 60
PI-DNA
Anti-GCSF SOmcg/nJ U 12.7%
Fig. 4. Study of growth stimulation by exogenous G-CSF administration and neutralization of growth-promoting activity by anli-G-CSF antibody. The proliferative activity of the
cultured cancer cells was measured by the flow cytometric BrdUrd incorporation technique. The double-stained cells were analyzed with the use of an Epics ELITE flow cytometer,
and the LI, i.e., the number of cells stained by BrdUrd divided by the total estimated cell count, was calculated. The Lis of the cells treated with 0.5, 0.1, and 0 |xg/ml (control) of
G-CSF were 16.7, 14.0, and 12.7%, respectively (A). For the neutralizing test, 0.5-fig/ml concentrations of G-CSF were preincubated with or without serial concentrations of anti-human
G-CSF antibody before their addition to the cultured cells. The experiment was otherwise carried out in exactly the same way as the stimulation test. As shown in B. the addition of
50 ng of anti-G-CSF antibody neutralized the cell growth stimulated by 0.5 (ig/ml of G-CSF, reducing growth by 26.2%.
statistically significantly lower than were those without anti-G-CSF
antibody cultures (P < 0.01).
Furthermore, when anti-G-CSF antibody was added in the cultures
every 24 h for three times, [3H]thymidine incorporations were
3750.8 ±178.8 cpm for 10 jig/ml, 3326.0 ±246.2 cpm for 50 fig/ml,
and 3166.7 ±113.0 cpm for 200 fig/ml anti-G-CSF antibody con
centrations. These were significantly lower than were those without
anti-G-CSF antibody administration (4132.2 ± 231.4 cpm/well;
P < 0.01). However, KU-7 cells did not demonstrate any inhibition of
BrdUrd labeling or [3H]thymidine incorporation when anti-G-CSF
antibody was cocultured.
Binding studies with the use of the radiolabeled recombinant G-
CSF demonstrated the presence of a high-affinity G-CSF binding
receptor on the cultured cancer cells (Fig. 6). Nonspecific binding
(binding of 125I-labeled KW-2228 to the cells in the presence of
G-CSF at 1000 ng/0.5 ml), which ranged between 6 and 18%, was
subtracted from the total binding to determine the specific binding.
The specific binding of the labeled KW-2228 was expressed as the
: .'X'-iif':
.;ri:VÕrifÃ-Ãœf"''0
10 20 30 40 50 $0
PI-DNA
Control LI 11.7%
li-
0 10 20 30 40 SO 60
PI-DNA
Anti-GCSF I0mcg/a/ LI 10.4%
^^i •'.•9
IO 20 30 40 SO 60
R-DNA
sV
°E
§Sf^*'"'f«ÕÜi.-J)
10 20 30 40 SO CO
PI-DNA
Anti-GCGF Wmct/mt LI 9.5» Anti-GCSF 200mcg/>J LI 8.9»
Fig. 5. Flow cytometric BrdUrd incorporation study on the effect of anti-G-CSF antibody on cell growth proliferation. Anti-G-CSF antibody was added in the cultures every 24 h
for three times under a serum-free condition. In vitro BrdUrd labeling was performed 24 h after the final administration of anti-G-CSF antibody. The LI was estimated as described
previously. BrdUrd Lis were 11.7, 10.4, 9.5, and 8.9% in anti-G-CSF antibody concentrations of 0, 10, 50, and 200 fig/ml, respectively.
3441
on July 8, 2017. © 1995 American Association for Cancer Research. cancerres.aacrjournals.org Downloaded from

AUTOCRINE CELL GROWTH WITH G-CSF
percentage of binding measured in the absence of unlabeled KW-2228
(Fig. 6A). The Scatchard plot analysis of the specific binding of
125I-labeled KW-2228 to the cells shown in Fig. 6ßindicates that the
cells harbor a single type of G-CSF receptor. The ßmaxcalculated
from the intercept of the slope with the abscissa on the Scatchard plot
was 458 molecules/cell, and the Kd was 103 pM.
DISCUSSION
Varieties of nonhematopoietic malignant tumors have been dem
onstrated to secrete G-CSF (2-11). In addition, it has been reported
that receptors for G-CSF have been confirmed on the cell surfaces of
several nonhematopoietic cell types (13-15). Bladder cancer cells
have been shown to secrete a variety of biological factors with no
direct relation to urothelial cell origin including G-CSF (2-4), GM-
CSF (2, 22), and various cytokines (22).
Previously, we have reported the expression of functional receptors
for G-CSF in transitional cell carcinoma of the bladder (16). In our
previous report, G-CSF receptors were expressed on two bladder
cancer cell lines, and administration of G-CSF provided increased cell
proliferation, as estimated by the [3H]thymidine incorporation
method.
These previous observations lead naturally to the tempting specu
lation that the simultaneous acquisition of G-CSF production and
expression of its receptor, by a malignant tumor, may enhance auto
crine growth. However, Sato et al. (4) reported on G-CSF-producing
bladder cancer, although they indicated that their study failed to
demonstrate a crucial role for G-CSF in mediating a growth advantage
for the tumor. Furthermore, Thacker et al. (23) demonstrated that the
human osteosarcoma cell line MG63 responds to both G-CSF and
GM-CSF in vitro. They indicated that retrovirally infected G-CSF or
GM-CSF-producing MG63 cells exhibited autostimulatory growing
features, as measured by [3H]thymidine incorporation.
Stimulation of cultured cancer cells by exogenous G-CSF admin
istration and neutralization of this growth-promoting activity by anti-
G-CSF antibody, as demonstrated by both the flow cytometric BrdUrd
incorporation technique and [3H]thymidine incorporation assay indi-
Table 1 Effects of G-CSF and anti-G-CSF antibody on [~H]thym\dine incorporation by
bladder cancer cells'
[' H]thymidine incorporation
(cpm/well)
G-CSF administration
Control 4760.6 ±309.1
0.1 Kg/ml G-CSF 5544.7 ±680.1
0.5 u.g/ml G-CSF 6030.8 ±409.5
G-CSF neutralization
Control (G-CSF 0.5 u,g/ml alone) 6180.8 ±285.3
0.5 ng/ml G-CSF + 10 /ig/ml anti-G-CSF Ab 4849.8 ±216.5
0.5 Mg/ml G-CSF + 50 /¿g/mlanti-G-CSF Ab 4373.0 ±278.1
Anti-G-CSF Ab administration
Control 4132.2 ±231.4
10 ng/ml anti-G-CSF Ab 3750.8 ±178.8
50 u,g/ml anti-G-CSF Ab 3326.0 ±246.2
200 ng/ml anti-G-CSF Ah 3166.7 ±113.0
"The uptake at 24-h incubation after the final G-CSF administrations of 0.1 and 0.5
¿ig/mlwere 5544.7 ±680.1 and 6030.8 ±409.5 cpm, respectively; these were significantly
higher than those in the controls (4760.6 ±309.1 cpm; P < 0.05). When 0.5 ug/ml G-CSF
was preincubated with serial concentrations of anti-G-CSF antibody, ["Hjthymidine
incorporations were 6180.8 ±285.3,4849.8 ±216.5, and 4373.0 ±278.1 cpm at 0, 10, and
50 fig/ml of anti-G-CSF antibody, respectively. ["Hjthymidine incorporation in cultures
with anti-G-CSF antibody was statistically significantly lower than was that in cultures
without anti-G-CSF antibody (P < 0.01). Furthermore, when anti-G-CSF antibody was
added in the cultures every 24 h for three times, ["Hjthymidine incorporation was
3750.8 ±178.8 cpm for 10 /xg/ml, 3326.0 ±246.2 cpm for 50 ug/ml, and 3166.7 ±113.0
cpm for 200 p.g/ml of anti-G-CSF antibody. These values were significantly lower than
were those without anti-G-CSF antibody administration (4132.2 ±231.4 cpm/well;
P < 0.01); Ab, antibody.
& 4
= 3
DO
.E o
â„¢1
î
A
B/F
0.3
0.2
0.1
10 20 30
'"l-G-CSF(ng)
40
10
B20 30
(pico mole)
40
Fig. 6. G-CSF receptor binding experiments. Nonspecific binding (binding of I25I-
labeled KW-2228 to the cells in the presence of G-CSF at 1000 ng/0.5 ml), which ranged
between 6 and 18%, was subtracted from the total binding to determine the specific
binding. The specific binding of the labeled KW-2228 was expressed as the percentage
of binding measured in the absence of unlabeled KW-2228 (A). The Scatchard plot
analysis of the specific binding of '25I-labeled KW-2228 to the cells shown in B indicates
that the cells harbor a single type of G-CSF receptor. The ßmaxcalculated from the
intercept of the slope with the abscissa on the Scatchard plot was 458 molecules/cell, and
the Ka was 103 pM.
cate that the proliferation of cultured cancer cells was stimulated by
G-CSF, and that this stimulation was inhibited by anti-G-CSF anti
body. In addition, binding studies performed with the use of radiola-
beled recombinant G-CSF demonstrated the presence of a high-
affinity G-CSF binding receptor on the cultured cancer cells.
These results strongly suggest that G-CSF production by the blad
der cancer cells produced an autocrine growth advantage. The leuke-
moid reaction is a well-known paraneoplastic syndrome that has been
demonstrated to be initiated by G-CSF production by cancer cells (1).
Furthermore, the leukemoid reaction has been widely observed clin
ically to appear at an advanced stage of cancer in association with
aggressive cell growth (4, 12). It is, therefore, deemed likely that the
G-CSF production and G-CSF receptor expression exhibited by can
cer cells play crucial roles in mediating the malignant progression of
the nonhematopoietic cancer cells.
The histogenesis of transitional cell carcinoma of the bladder re
mains uncertain, although several theories have been proposed. Some
authors have suggested that a metaplastic phenomenon presenting
various degrees of differentiation may explain the malignant transi
tional cell G-CSF production (24).
In addition, this concept is supported further by the tremendous
potential of the transitional epithelium and transitional cell carcinoma
to differentiate along several lines (25). The frequent presence of both
squamous and glandular differentiation has long been recognized in
transitional cell carcinoma. More recently, the presence of neuroen
docrine (small cell) differentiation has also been reported (26). He-
matopoietic differentiation of transitional cell carcinoma, resulting in
acquisition of G-CSF production and G-CSF receptor expression, is
another possibility supported by our observations.
3442
on July 8, 2017. © 1995 American Association for Cancer Research. cancerres.aacrjournals.org Downloaded from
 6
6
 7
7
1
/
7
100%