corrigé

DS
SECONDE
THÈME UNIVERS
L’ATOME ET LES ÉLÉMENTS CHIMIQUES DANS LA CLASSIFICATION PÉRIODIQUE
!"#$%&%'%(!
NOM : .......................... PRÉNOM : ................... CLASSE : ............. DATE : .................................
%
Exercice''1'(8'points)'(8,00)
40,0
Donnée':'
" )*"+#$%,-$).+/01$%,-,2$3."/+$4%$%5%&467%8%&79&:%;%
<=/.%13%".=2$%>$%?-"./3$%@PtAB%C$%3=D"1%>$%)$.%".=2$%)=3./$3.%&:E%31)-,=3F%$.%F"%)*"+#$%
,-$).+/01$%.=."-$%G%$F.%,#"-$%H%I%&4(JK%8%&79&L%;B%
&B G1$--$%$F.%-"%)*"+#$%,-$).+/01$%?=+.,$%?"+%-$%31"#$%,-$).+=3/01$%>$%)$.%".=2$%>$%?-"./3$%M%
CN".=2$%$F.%3$1.+$%,-$).+/01$2$3.%F"%)*"+#$%.=."-$%G.=."-$%$F.%>=3)%31--$%
𝑄!"!#$% =𝑄!"#$% +𝑄!"!#$%&'( =0!𝐶%
𝑄!"!#$%&'( =!−!𝑄!"#$%%
𝑄!"!#$%&'( =!1,248!x!10!!"!C'
2
(B O,.$+2/3$+%$F.%F=3%312,+=%".=2/01$%P%
𝑄!"#$% =𝑍.𝑒%
𝑍=𝑄!"#$%
𝑒%
𝑍=
1,248!×!10!!"
1,60×10!!!%
𝑍=78%
-N".=2$%?=FFQ>$%LK%?+=.=3F%>"3F%F=3%3=D"1%
2
RB G1$-%$F.%F=3%3=2S+$%>$%3$1.+=3F%M%
𝑁=𝐴−𝑍%
𝐴=195!%
𝑁=195 −78%
𝑁=117%
T-%D%"%>=3)%&&L%3$1.+=3F%>"3F%-$%3=D"1%>$%)$.%".=2$%
2
JB G1$-%$F.%-$%FD2S=-$%>$%F=3%3=D"1%M%
C$%FD2S=-$%>$%)$.%".=2$%$F.%>=3)% 𝑃𝑡
!"
!"# %
2
Exercice'2'(7'points)'7,00'
Données':'
9%%2"FF$%>N13%?+=.=3%U%2?%5%&46LR%8%&79(L%V#%W%
9%2"FF$%>N13%3$1.+=3%U%23%5%&46LE%8%&79(L%V#%W%
9%&%"33,$%5%R&%EEL%677%F$)=3>$FB'
&B XY"-1$+%-"%2"FF$%2@Z-A%>N13%".=2$%>N"-12/3/12%@P5&R%W%Z%5%(LAB%C$%+,F1-.".%>$Y+"%[.+$%$8?+/2,%
"Y$)%%J%)*/\\+$F%F/#3/\/)"./\FB%
%
C$%3=D"1%>$%-N".=2$%>N"-12/3/12%)=3./$3.%%
P%?+=.=3F%$.% 𝐴−𝑍!3$1.+=3F%%$.%P%,-$).+=3F%
-"%2"FF$%>$F%,-$).+=3F%$F.%3,#-/#$"S-$%>$Y"3.%-"%2"FF$%>$F%31)-,=3F%%
C"%2"FF$%>$%-N".=2$%>N"-12/3/12%F$%+,>1/.%H%U%
𝑚!" =𝑍.𝑚!"#$#%&!+𝐴−𝑍.𝑚!"#$%&!'!%
𝑚!" =13×1,673.10!!" !+27 −13 ×1,675.10!!"%
𝑚!" =45,20!.10!!"𝑘𝑔%
2

(B ]3%,)*"3./--=3%>N"-12/3/12%2,."--/01$%H%?=1+%2"FF$%^%5%(L47%#B%
(B&B ;=2S/$3%)$.%,)*"3./--=3%)=3./$3.9/-%>N".=2$F%>N"-12/3/12%M%
𝑁
!" =
𝑀
𝑚!"
%
𝑀=27,0!𝑔%
𝑚!" =45,20!.10!!!𝑔%
𝑁
!" =
27,0
45,20!.10!!!%
𝑁
!" =5,97!.10!"𝑎𝑡𝑜𝑚𝑒𝑠%
>"3F%(L47%#%>N"-12/3/12 𝐴𝑙
!"
!" %+$3\$+2$3.%%$3Y/+=3%</8%)$3.F%2/--$F%>$%2/--/"+>F%>$%2/--/"+>F%
>N".=2$F%>N"-12/3/12%
2
(B(B ;=2S/$3%>N"33,$F%\"1>+"/.9/-%?=1+%)=2?.$+%.=1F%)$F%".=2$F%>N"-12/3/12%H%+"/F=3%>$%13%?"+%
F$)=3>$%M%;=2?"+$+%)$..$%Y"-$1+%H%)$--$%>$%-N_#$%>1%FDF.Q2$%F=-"/+$%$F./2,$%H%E%8%&7:%"3F%@E%
2/--/"+>F%>N"33,$FA%
%
%
</%=3%)=2?.$%"1%+D.*2$%@Y%5%&%".=2$BF9&A%>N13%".=2$%?"+%F$)=3>$%/-%\"1.%13$%>1+,$%∆𝑡%
∆𝑡=
𝑁
!"
𝑣%
∆𝑡=
5,97!.10!"
1%
∆𝑡=5,97!.10!"!𝑠%
=+%%
1an!=!365,25×24×3600 =𝟑𝟏!𝟓𝟓𝟕!𝟔𝟎𝟎!𝑠!
∆𝑡=
5,97!.10!"!𝑠
𝟑𝟏!𝟓𝟓𝟕!𝟔𝟎𝟎!%
∆𝑡=1,89.10!"!𝑎𝑛𝑛é𝑒𝑠%
F=/.%$3Y/+=3%(7%2/--/=3F%>$%2/--/"+>F%>N"33,$F%
)=2?"+"/F=3%H%-N_#$%>1%FDF.Q2$%F=-"/+$%
1,89.10!"!
5.10!!=!4.10!𝑎𝑛𝑠%
𝑖𝑙!𝑓𝑎𝑢𝑡𝑑𝑟𝑎𝑖𝑡!𝑒𝑛𝑣𝑖𝑟𝑜𝑛!4!𝑚𝑖𝑙𝑙𝑖𝑜𝑛𝑠!𝑑𝑒!𝑓𝑜𝑖𝑠!𝑙!â𝑔𝑒!𝑑𝑢!𝑠𝑦𝑠𝑡ème$solaire$pour$pouvoir$finir$le$décompte$$
de$ces$atomes$
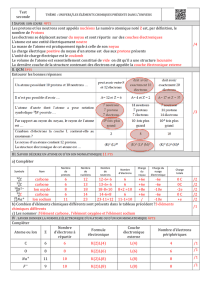
Exercice'4'(8'points)'12,00)%
2
1
'
Symbole'de'
l’atome'ou'
de'l’ion'
O'
Li+'
Si'
Ca2+'
Au'
Br'I'
𝐅𝐞𝟐!'
Al3+'
Symbole'du'
noyau'
O
17
8
%
Li
7
3
%
Si
28
14
%
Ca
40
20
%
Au
197
79
%
Br
80
35
%
26
56 Fe
%
𝐀𝐥
𝟏𝟑
𝟐𝟕
%
Nombre'de'
protons'
K%
R%
&J%
(7%
L:%
RE%
(6%
&R%
Nom're'de'
neutrons'
:%
J%
(K9&J5%
&J%
J79(75%
(7%
&:L9
L:5%
&&K%
K79RE5%
JE%
E69(65%
R7%
&J%
Nombres'
d’électrons'
K%
(%
&J%
(79
(5&K%
L:%
R6%
(J%
&7%
12
Exercice'5'(13'points)'13,00'
'

`3%)=3F/>Q+$%13%".=2$%>$%2"#3,F/12%@MgA%>$%312,+=%".=2/01$%P%5%&(B%
%
&B O=33$+%-"%\=+21-$%,-$).+=3/01$%>$%-N".=2$%>$%2"#3,F/12%
Puisqu’il's’agit'de'l’atome'il'y'a'12'électrons'à'répartir':'K(2)L(8)M(2)'
1
(B a3%>,>1/+$%F"%?-")$%>"3F%-"%)-"FF/\/)"./=3%?,+/=>/01$%@?,+/=>$%$.%)=-=33$AB%
P5&(%/-%FN"#/.%>1%>=1b/Q2$%,-,2$3.%>$%-"%)-"FF/\/)"./=3%
C"%)=1)*$%$8.$+3$%$F.%-"%)=1)*$%^%-N,-,2$3.%$F.%>=3)%F1+%-"%.+=/F/Q2$%?,+/=>$%
T-%+$3\$+2$%(%,-$).+=3F%?,+/?*,+/01$F%-N,-,2$3.%$F.%>=3)%F/.1,%F1+%-"%>$18/Q2$%)=-=33$%@\"2/--$%
>$F%2,."18%"-)"-/3=9.$++$18%
%
2
RB Z%01$--$%+Q#-$4%-N".=2$%>$%2"#3,F/12%=S,/.9/-%$3%\=+2"3.%13%/=3%M%X3=3)$+%)$..$%+Q#-$B%
!=1+%#"#3$+%$3%F."S/-/.,%13%".=2$%?$1.%F$%.+"3F\=+2$+%$3%/=3%2=3=".=2/01$%F"./F\"/F"3.%"/3F/%H%-"%
+Q#-$%>$%-N=).$.%@=1%>1%>1$.A%
!=1+%#"#3$+%$3%F."S/-/.,%13%".=2$%,Y=-1$%>$%\"c=3%H%")01,+/+%13$%F.+1).1+$%,-$).+=3/01$%
F$2S-"S-$%H%-"%F.+1).1+$%,-$).+=3/01$%>1%#"b%3=S-$%>$%312,+=%".=2/01$%-$%?-1F%?+=)*$%>"3F%-"%
)-"FF/\/)"./=3%@-"%)=1)*$%$8.$+3$%")01/$+.%"/3F/%13$%F.+1).1+$%$3%=).$.%=1%>1$.%@K%=1%(%,-$).+=3F%@F/%
)=1)*$%dA%%?,+/?*,+/01$A%%
2
JB G1$--$%$F.%-"%\=+21-$%)*/2/01$%>$%-N/=3%01$%?$1.%>=33$+%-N".=2$%>$%2"#3,F/12M%
C$%2"#3,F/12%"%>$18%,-$).+=3F%?,+/?*,+/01$F%F1+%-"%)=1)*$%^%/-%?$+>%\")/-$2$3.%)$F%>$18%
,-$).+=3F%?=1+%01$%-"%)=1)*$%$8.$+3$%%>$Y/$33$%-"%)=1)*$%CH%%K%,-$).+=3FB%%
%
T-%"1+"%"/3F/%-"%2[2$%)=3\/#1+"./=3%,-$).+=3/01$%01$%-$%e$=3%01/%$F.%-$%#"b%3=S-$%-$%?-1F%%?+=)*$%
>1%2"#3,F/12%>"3F%-"%)-"FF/\/)"./=3%?,+/=>/01$F%
%
<"%\=+21-$%)*/2/01$%F$+"%U!𝐌𝐠𝟐!'et'sa'formule'électronique'K(2)L(8)%
2
EB G1$--$%$F.%-"%\=+21-$%,-$).+=3/01$%>$%-N".=2$%>$%S,+D--/12%@BeA%F/.1,%f1F.$%auIdessus%>1%
2"#3,F/12%>"3F%-"%)-"FF/\/)"./=3%?,+/=>/01$B%%
;$.%,-,2$3.%$F.%F/.1,%13$%-/#3$%"1%>$FF1F%>1%2"#3,F/12%F"%)=1)*$%$8.$+3$%$F.%-"%)=1)*$%C%
]3%,-,2$3.%F/.1,%"1%>$FF1F%>1%2"#3,F/12%"1+"%-"%2[2$%)=3\/#1+"./=3%,-$).+=3/01$%$8.$+3$%01$%
)$-1/%)/%)"+%/-%%"??"+./$3.%H%-"%2[2$%\"2/--$%)*/2/01$%T-%+$3\$+2$%(%,-$).+=3F%?,+/?*,+/01$F%F1+%-"%
)=1)*$%$8.$+3$%d%
<"%\=+21-$%,-$).+=3/01$%$F.%>=3)%K(2)L(2)'
Équation'de'formation'de'l’ion'Béryllium'
𝑩𝒆 →!𝐁𝐞𝟐!+𝟐𝒆!'
%
2
6B CN,-,2$3.%%)*-=+$%"??"+./$3.%H%-"%&LQ2$%)=-=33$%@gTTA%>$%-"%)-"FF/\/)"./=3%?,+/=>/01$B%
6B&B Z%01$--$%\"2/--$%"??"+./$3.9/-%M%
;$.%,-,2$3.%"??"+./$3.%H%-"%\"2/--$%>$F%*"-=#Q3$F%
2
6B(B G1$--$%$F.%-"%\=+21-$%>$%-N/=3%01$%?$1.%\=+2$+%-N".=2$%>$%)*-=+$%M%
h=1F%-$F%i"-=#Q3$F%=3.%F$?.%,-$).+=3F%?,+/?*,+/01$F%?=1+%#"#3$+%$3%F."S/-/.,%/-F%?$1Y$3.%F$%
.+"3F\=+2$+%$3%/=3%2=3=".=2/01$%"D"3.%13%,-$).+=3F%F1??-,2$3."/+$%"\/3%>N"Y=/+%13$%
)=3\/#1+"./=3%,-$).+=3/01$%$8.$+3$%H%K%,-$).+=3F%?,+/?*,+/01$FB%;$%\"/F"3.%/-F%\=+2$%13%/=3%
2=3=".=2/01$%?=+.$1+%>N13$%)*"+#$%,-,2$3."/+$%3,#"./Y$B%C$%)*-=+$%\=+2$+"%>=3)%-N/=3%)*-=+1+$%
!𝐂𝐥!!𝒔𝒂!𝒄𝒐𝒖𝒄𝒉𝒆!𝒆𝒙𝒕𝒆𝒓𝒏𝒆!𝒅𝒆!𝒍!𝒊𝒐𝒏!𝒄𝒉𝒍𝒐𝒓𝒖𝒓𝒆!𝒑𝒐𝒔𝒔è𝒅𝒆𝒂𝒍𝒐𝒓𝒔!𝟖!é𝒍𝒆𝒄𝒕𝒓𝒐𝒏𝒔!𝒑é𝒓𝒊𝒑𝒉é𝒓𝒊𝒒𝒖𝒆𝒔'
Équation'de'formation'de'l’ion'chlorure'
𝑪𝒍 +𝒆!→!𝐂𝐥!'
'
1

6BRB CN,-,2$3.%2"#3,F/12%$.%-N,-,2$3.%)*-=+$%?$1Y$3.%\=+2$+%-$%)=2?=F,%)*/2/01$%>$%\=+21-$%
^#;-(%@)*-=+1+$%>$%2"#3,F/12AB%%
G1$--$%$F.%-"%\=+21-$%)*/2/01$%>1%)=2?=F,%)*/2/01$%01$%-N,-,2$3.%S,+D--/12%@BeA%?$1.%
\=+2$+%"Y$)%-N,-,2$3.%)*-=+$%M%
O"3F%-$%)*-=+1+$%>$%2"#3,F/12%-N/=3%2"#3,F/12%$F.%F=1F%-"%\=+2$%!𝐌𝐠𝟐!%C$%j,+D--/12%%\=+2$+"%>=3)%
)=22$%-$%2"#3,F/12%13%/=3%2=3=".=2/01$%"Y$)%>$18%)*"+#$F%,-,2$3."/+$F%?=F/./Y$4%-"%\=+21-$%
>$%-N/=3%\=+2,%$F.%>=3)!𝐁𝐞𝟐!%
C$%)*-=+1+$%>$%j,+D--/12%\=+2$+"%13%,>/\/)$%/=3/01$%01/%$F.%#-=S"-$2$3.%3$1.+$%-$F%>$18%)*"+#$F%?=F/./Y$F%
?=+.,$F%?"+%-N/=3%%!𝐁𝐞𝟐!F=3.%)=2?$3F,$F%?"+%>$18%/=3F%)*-=+1+$!𝐂𝐥!C"%\=+21-$%>1%)*-=+1+$%>$%S,+D--/12%
$F.%F$2S-"S-$%H%)$--$%>1%)*-=+1+$%>$%2"#3,F/12%F=/.%j$;-(%
1
%
1
/
4
100%