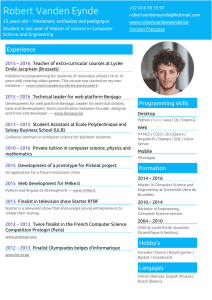
Corporate Presentation 2016

Corporate
Presentation
2016

2
Presentation 2016
IMPORTANT NOTICE AND DISCLAIMER
Important: You must read the following before continuing. By accessing the information in this document and by attending any oral presentation made in
conjunction with this document you represent, warrant and undertake that you have read and agreed to comply with the contents of this Notice and Disclaimer.
This document, the oral presentation accompanying this document and any questions and answers session following the oral presentation (the “Presentation”) have been
prepared by Nanobiotix (the “Company”) solely for selected qualified institutional buyers (“QIBs”) as defined in Rule 144A of the Securities Act of 1933, as amended (the
“Securities Act”) for information purposes. The information contained in the Presentation is strictly confidential and may not be copied, reproduced, distributed or disseminated,
directly or indirectly, in whole or in part, by or to any person.
This document does not purport to contain comprehensive or complete information about the Company and is qualified in its entirety by the business, financial and other
information that the Company is required to publish in accordance with the rules, regulations and practices applicable to companies listed on Euronext Paris, including the risk
factors, in the Company’s Document de référence (Registration Document), registered by the AMF on January 27, 2014 under no. R.14-0002, and in any other periodic report,
which are available free of charge on the websites of the Company (www.nanobiotix.fr) and the AMF (www.amf-france.org). Certain figures and numbers appearing in this
document have been rounded. Consequently, the total amounts and percentages appearing in tables and elsewhere may not necessarily equal the sum of the individually
rounded figures, amounts or percentages.
No representation, warranty or undertaking, express or implied, is made as to the accuracy, completeness or appropriateness of the information and opinions contained in this
Presentation, or its use for any purpose, and no reliance should be placed on any information or opinions contained in it. The Company, its subsidiaries, its advisors and
representatives accept no responsibility for and shall not, under any circumstance, be held liable for any loss or damage that may arise from the use of this Presentation or the
information or opinions contained in it. In particular, this document contains information on the use of the Company products and its competitive position. This information has
been drawn from various sources or from the Company’s own estimates which may not be accurate and thus no reliance should be placed on such information. Any
prospective investors must make their own investigation and assessments and consult with their own advisers concerning any evaluation of the Company and its prospects, and
this Presentation, or any part of it, may not form the basis of or be relied on in connection with any investment decision.
All statements in the Presentation other than statements of historical fact are or may be deemed to be forward-looking statements. These forward looking statements can be
identified by the use of forward looking terminology, including the terms “will”, “should”, “develop”, “development”, “strategy”, “targeting”, “expanding”, “mid term”, “medium term”
“enable”, “anticipated”, “expected”, “benefits”, “expand”, “target”, “promising”, “plan”, “evolve”, “optimize”, “prepare”, “pipeline”, “grow”, “time”, “timeline”, “endpoints”, “potential”,
“route”, “trend”, “grow”, or other forward looking vocabulary and terminology, or by discussions of strategy, plans, objectives, goals, expectations, hopes, future events,
operations or intentions, estimates and projections with respect to the Company’s products and markets for its products. These forward-looking statements are not guarantees of
future performance and involve a number of known and unknown risks and uncertainties. These risks and uncertainties, and other factors, could adversely affect the outcome of
the forward looking statements, and actual results could differ materially from those contemplated in the statements. As a result, you are cautioned not to rely on such forward-
looking statements. Forward-looking statements speak only as to the date of this document and the Company expressly disclaims any obligation or undertaking to update or re-
issue any forward-looking statements contained in this Presentation.
The information contained in this Presentation is provided as of the date of this document only and may be updated, supplemented, revised or amended, and thus such
information may be subject to changes at any time. Neither the Company, nor its advisors, nor any other person is under any obligation to update the information, statements or
opinions contained in this Presentation.
This Presentation does not constitute or form any part of any offer to sell, or the solicitation of an offer to buy or subscribe for, any shares or securities in the Company in the
United States or in any other jurisdiction. Securities may not be offered or sold in the United States absent registration or an exemption from registration under the U.S.
Securities Act of 1933, as amended. The Company has not registered, and does not intend to register, any offering of its securities in the United States.

3
Presentation 2016
NanoXray positioned to become a standard of care
in oncology
First in class radioenhancer that could change everything without
changing anything
Lead product has shown to be both safe and efficacious in “ultimate tumor case”
Derisking the all concept
Allows for higher dose/efficacy without increasing toxicity
1
Disruptive innovation compatible with care in current hospital setting
Disruptive innovation in existing and well structured radiotherapy market
Does not require change of the existing infrastructure, protocol, training
3
Several key milestones in the coming 12 months
Sarcoma Phase II/III interim data mid 2016 and CE mark end of 2016
Data in Liver cancer Phase I/II starting H2 2016
Results of Phase I/II H&N cancer H1 2016
4
Versatile application to target the majority of the oncology market
Broad pipeline in six major indications with potential expansion in most of solid tumors
NanoXray can easily be combined with chemotherapy, immuno-oncology …
2

4
Presentation 2016
Nanobiotix applies physics to cancer treatment
Physics
Biology
Chemistry Nanosized drug
delivery system
Biology
1940’s 1980’s 2000
e.g. Mechlorethamine e.g. Herceptin
New therapeutic
tools based on
physics
e.g. Doxil e.g. NanoXray
Molecules
(drugs and biologics)
Nanotechnology
supports the effect of
molecules
Nano-object becomes
the active principle
Today
50nm

5
Presentation 2016
Company Snapshot
General description History
2003: Founding of Nanobiotix
2012: $57m plus royalties collaboration with PharmaEngine
for NBTRX3 in Asia
2012: Listing on NYSE Euronext Paris
2014: Positive safety and efficacy results of NBTRX3 in STS
2015: Start of phase I/II NBTXR3 in liver mets and HCC
NanoXray
Nanobiotix is a French company headquartered in Paris and
has US affiliate in Cambridge
Nanobiotix develops enhanced radiotherapy through
nanotechnology to treat cancer
Market cap on NYSE Euronext Paris is € 230 m*
Well funded: €25m of cash & cash equivalents**
Nanobiotix develops innovative nanoparticle technology
dedicated to the local treatment of cancer
Nanoparticles interact with radiotherapy and maximize the
effect of radiotherapy within tumors
Lead product NBTXR3 targets several major cancers
NBTXR3 first registration study in STS (EU, Asia, Can) has
started targeting 2016 for first approval
* As of December 2015 (Bloomberg); ** As of end 2015 H1
 6
6
 7
7
 8
8
 9
9
 10
10
 11
11
 12
12
 13
13
 14
14
 15
15
 16
16
 17
17
 18
18
 19
19
 20
20
 21
21
 22
22
 23
23
 24
24
 25
25
 26
26
 27
27
 28
28
 29
29
 30
30
 31
31
 32
32
 33
33
 34
34
1
/
34
100%