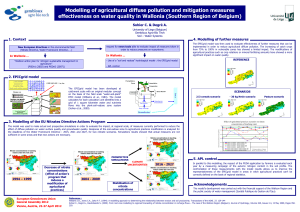
Test for Cations and Anions in
Aqueous Solutions
November 30, 2020 by Veerendra
Test for Cations and Anions in Aqueous
Solutions
Test for anions in aqueous solutions
1. When a salt is dissolved in water, the free anion will be present in the aqueous solution.
Tests can then be carried out to identify the anion.
2. The following shows the various conrmatory tests for carbonate ion, chloride ion,
sulphate ion and nitrate ion in aqueous solutions.
Test for carbonate ion, CO
Method:
Add dilute hydrochloric acid.
Bubble gas through limewater.
32-
MENU

Observation:
Limewater turns milky.
What happened?
Acids react with carbonate ion to produce carbon dioxide gas.
CO (aq) + 2H (aq) → CO (g) + H O(l)
Carbon dioxide turns limewater milky due to the formation of calcium carbonate (white
precipitate).
CO (g) + Ca(OH) (aq) → CaCO (s) + H O(l)
Test for chloride ion, Cl
Method:
Add dilute nitric acid.
Then add silver nitrate solution.
Observation:
A white precipitate is obtained.
What happened?
Silver ion, Ag , from silver nitrate combines with chloride ion, Cl , to form the silver
chloride.
Ag (aq) + Cl (aq) → AgCl(s)
Silver chloride is an insoluble salt and forms as a white precipitate.
32- + 2 2
2 2 3 2
–
+ –
+ –

The nitric acid added is to prevent precipitation of silver sulphate and silver carbonate.
Test for sulphate ion, SO
Method:
Add dilute hydrochloric acid.
Then, add barium chloride solution.
Observation:
A white precipitate is obtained.
What happened?
Barium ion, Ba , from barium chloride combines with the sulphate ion, SO , to form
barium sulphate.
Ba (aq) + SO (aq) → BaSO (s)
Barium sulphate is an insoluble salt and forms as a white precipitate.
The hydrochloric acid added is to prevent precipitation of barium carbonate.
Test for nitrate ion, NO
Method:
42-
2+ 42-
2+ 42- 4
3–

Add dilute sulphuric acid.
Then, add iron(II) sulphate solution.
Shake to mix well.
Carefully add concentrated sulphuric acid down the side of the test tube.
Observation:
Brown ring is obtained.
What happened?
Concentrated sulphuric acid reacts with the nitrate ion to form nitrogen monoxide
molecule, NO.
Nitrogen monoxide combines with iron(II) sulphate to form a brown complex which
appears as a brown ring.
This test is also known as the ‘brown ring test’.
People also ask
Classication of Salts
General Properties of Salts
Uses of different salts in daily life
Preparation of Salts
Describe the preparation of soluble and insoluble salts
Qualitative Analysis of Salts
Action of Heat on Salts

Constructing ionic equations using the continuous variation method
What is stoichiometry and why is it used in chemistry?
Test for anions examples
1.Some tests were carried out on salt P. The results obtained were shown below.
Test Observation
1. Salt P was heated.
No residue was left in the test tube.
A gas was evolved which turned red litmus
paper blue.
2. Salt P was dissolved in water.
Dilute nitric acid was added, followed by
silver nitrate solution.
A white precipitate was formed.
Identify salt P.
Solution:
The gas liberated is ammonia because it is an alkaline gas. Hence, ammonium ion is present.
Anion is a chloride ion because silver chloride is precipitated. Salt P is ammonium chloride.
2.Figure shows the reaction scheme of a compound Q.
Identify compound Q.
Solution:
The gas is carbon dioxide. Q contains a carbonate ion. Zinc oxide is yellow when hot and
white when cold. Hence, Q is zinc carbonate.
 6
6
 7
7
 8
8
 9
9
 10
10
 11
11
 12
12
 13
13
 14
14
 15
15
 16
16
 17
17
 18
18
 19
19
 20
20
1
/
20
100%