Markhamia tomentosa Antiprotozoal Activity: Scientific Study
Telechargé par
bernard.weniger

Antiprotozoal activities of some constituents of
Markhamia tomentosa (Bignoniaceae)
F. TANTANGMO
*
, B. N. LENTA
{
, F. F. BOYOM
{
, S. NGOUELA
*
, M. KAISER
1
,
E. TSAMO
*
, B. WENIGER
"
, P. J. ROSENTHAL
**
and
C. VONTHRON-SE
´NE
´CHEAU
",{{
*
Department of Organic Chemistry, Faculty of Science, TWAS Research Unit of the University of
Yaounde´ I, P.O. Box 812, Yaounde´, Cameroon
{
Department of Chemistry, Higher Teachers’ Training College, University of Yaounde´ I, P.O. Box
47, Yaounde´, Cameroon
{
Department of Biochemistry, Faculty of Science, University of Yaounde´ I, P.O. Box 812,
Yaounde´, Cameroon
1
Swiss Tropical and Public Health Institution, Socinstrasse 57, CH-4002 Basel, Switzerland
"
Laboratoire de Pharmacognosie et Mole´cules Naturelles Bioactives, UMR 7200, Faculte´de
Pharmacie, Universite´ de Strasbourg, B.P. 60024, 67401, Illkirch Cedex, France
**
Division of Infectious Diseases, Department of Medicine, University of California, San
Francisco, 1001 Portero Avenue, San Francisco, CA 94943, U.S.A
{{
Laboratoire de Biologie et de Biotechnologies Marines, UMR M IFREMER 100, Universite´de
Caen Basse-Normandie, Esplanade de la Paix, 14032 Caen Cedex, France
Received 10 May 2010, Accepted 17 May 2010
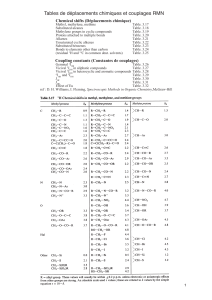
Phytochemical investigation of an ethyl-acetate extract of the stem bark of Markhamia tomentosa (Bignoniaceae),
which had good antimalarial activity in vitro, resulted in the isolation of eight known compounds: 2-
acetylnaphtho[2,3-b]furan-4,9-dione (1), 2-acetyl-6-methoxynaphtho[2,3-b]furan-4,9-dione (2), oleanolic acid
(3), pomolic acid (4), 3-acetylpomolic acid (5), tormentic acid (6), b-sitosterol (7) and b-sitosterol-3-O-b-D-
glucopyranoside (8). The structures of these compounds were established by spectroscopic methods. Each of
compounds 1,2,4and 5was evaluated in vitro for its antiprotozoal activities against the ring stages of two
chloroquine-resistant strains of Plasmodium falciparum (K1 and W2), the amastigotes of Leishmania donovani, and
the bloodstream trypomastigotes of Trypanosoma brucei rhodesiense (the species responsible for human malaria,
visceral leishmaniasis and African trypanosomiasis, respectively). Although compounds 1and 2exhibited potent
antiprotozoal activities, they also showed high toxicity against a mammalian (L-6) cell line.
Plants of the genus Markhamia are widely
distributed in Africa, where they are used
for the treatment of several human diseases
(Kerharo, 1978; Letouzey, 1982; Adjanouhoun
et al., 1996). In Tanzania, for example,
aqueous extracts of the root bark of M. lutea
are used to treat anaemia and diarrhoea
(Kerharo, 1978; Adjanouhoun et al., 1996).
In Cameroon, M. lutea and M. tomentosa
are both used to cure various microbial
and parasitic diseases (Adjanouhoun et al.,
1996). In previous phytochemical studies of
Markhamia spp, bio-active lignans, phenyl
glycosides, anthraquinones, alkaloids, phenyl
propanoids and terpenoids have been isolated
(Adesanya and Nia, 1997; Kernan et al., 1998;
Khan and Mlungwana, 1999; Kanchanapoom
et al., 2002; Lacroix et al., 2009). As part of
an ongoing investigation of antiprotozoal
agents in Cameroonian medicinal plants, an
Reprint requests to: C. Vonthron-Se´ne´cheau.
E-mail: [email protected].
Annals of Tropical Medicine & Parasitology, Vol. 104, No. 5, 391–398 (2010)
#W. S. Maney & Son Ltd 2010
DOI: 10.1179/136485910X12743554760180

ethyl-acetate extract of M. tomentosa was
prepared and found to have good antimalar-
ial activity in vitro against two strains (K1
and W2) of Plasmodium falciparum (see
below). Chromatographic fractionation of
this extract led to the isolation of eight
known compounds: 2-acetylnaphtho[2,3-b]
furan-4,9-dione (1), 2-acetyl-6-methoxy-
naphtho[2,3-b]furan-4,9-dione(2), oleanolic
acid (3), pomolic acid (4), 3-acetylpomolic
acid (5), tormentic acid (6), b-sitosterol (7)
and b-sitosterol-3-O-b-D-glucopyranoside (8).
The preparation of three extracts of M.
tomentosa stem bark, the isolation of com-
pounds 1–8from the ethyl-acetate extract,
and evaluation of the in-vitro activities of the
extracts and some of the isolated com-
pounds, against strains of Plasmodium,
Leishmania and Trypanosoma and a mamma-
lian cell line, are described below.
MATERIALS AND METHODS
General
Melting points were determined on an M-540
melting-point unit (Bu¨ chi, Flawil, Switzerland).
Optical rotations were measured, in chloro-
form solution, on a DIP-3600 digital polari-
meter (JASCO, Tokyo). Infra-red (IR) spectra
were determined on a Fourier transform
IR spectrometer (Jasco) while ultra-violet
(UV) spectra were determined on a Unicam
spectrophotometer (Spectronic Analytical
Instruments, Leeds, U.K.).
1
H and
13
C
nuclear-magnetic-resonance (NMR) spectra
were investigated on a Bruker spectrometer
(Bruker Optik, Ettlingen, Germany) equipped
with 5-mm
1
Hand
13
C probes operating at
500 and 125 MHz, respectively, with tetra-
methylsilane as the internal standard. Silica
gels of 230- to 400-mesh and 70- to 230-mesh
(Merck, Darmstadt, Germany) were used for
flash and column chromatography, respec-
tively, while aluminium sheets precoated
with silica gel 60 F
254
(Merck) were used
for thin-layer chromatography (TLC), with
various mixtures of petroleum ether, n-
hexane, ethyl acetate, and acetone as eluents.
Spots were visualized with UV light (at 254
and 365 nm) or using methanol–H
2
SO
4
reagent.
Plant Material
The stem bark of M. tomentosa was collected
in June 2007 at Mont Eloundem (Yaounde´)
in the Central province of Cameroon. The
plants were identified by N. Victor, a
botanist at the National Herbarium of
Cameroon, where a voucher specimen
(6475/SRF/Cam) was deposited.
Extraction and Isolation
The dried stem bark (4 kg) of M. tomentosa
was extracted successively with hexane,
ethyl acetate and methanol, by maceration.
In each extraction, two 10-litre volumes of
solvent were used over a period of 48 h.
After concentration under vacuum at room
temperature, a green hexane (9 g), a brown
ethyl-acetate (50 g) and a brown methanolic
extract (100 g) were produced. When each
of these extracts was screened in vitro for its
activities against the W2 and K1 strains of
Plasmodium falciparum, the ethyl-acetate
extract was found to have the highest
antimalarial activity against both strains
(see below). This extract was therefore
fractionated by column chromatography on
silica gel (230- to 400-mesh), with n-
hexane–ethyl-acetate mixtures of increasing
polarity being used as the eluents. Ninety
fractions, each of 400 ml, were collected
and combined, on the basis of the results of
TLC, to yield four main fractions that were
labelled F
1
(4.0 g), F
2
(7.1 g), F
3
(9.3 g)
and F
4
(11.7 g).
Fraction F
1
(4.0 g) was essentially an oil
that was not further investigated. Fraction
F
2
(7.1 g) was subjected to column chro-
matography over silica gel (70- to 230-
mesh), eluting with n-hexane–ethyl-acetate
gradient mixtures. This resulted in the
collection of 76 sub-fractions, each of
150 ml, which were combined on the basis
of the results of TLC analysis. Further
purification of sub-fractions 40–45 afforded
392 TANTANGMO ET AL.

b-sitosterol 7(300 mg) and 2-acetyl-
naphtho[2,3-b]furan-4,9-dione 1(30 mg).
Sub-fractions 53–55 yielded 2-acetyl-6-
methoxynaphtho[2,3-b] furan-4,9-dione 2
(35 mg). Successive chromatography of frac-
tions 68–70 afforded oleanolic acid 3
(625 mg). Fraction F
3
(9.3 g) was also sub-
jected to column chromatography over silica
gel (70- to 230-mesh), eluting with n-hexane–
ethyl-acetate mixtures (80 : 20–75 : 25) to
yield pomolic acid 4(200 mg) and a
powder of a mixture of compounds
(400 mg). The powder was rechromato-
graphed, using silica gel (70- to 230-mesh)
and eluting with dichloromethane–metha-
nol mixtures (99 : 1–98.5 : 1.5), to yield 3-
acetylpomolic acid 5(300 mg), tormentic
acid 6(30 mg) and b-sitosterol-3-O-b-D-
glucopyranoside 8(600 mg). Fraction F
4
(11.7 g) was another complex mixture
that was not studied further.
Assays of Biological Activity
ANTIMALARIAL ACTIVITY (W2 STRAIN)
Antimalarial activity was first determined,
in vitro, using the W2 strain of P. falciparum,
which is resistant to chloroquine and
some other antimalarial drugs (Singh and
Rosenthal, 2001). The parasites were cul-
tured in sealed flasks at 37uC, in an atmo-
sphere containing 3% (v/v) O
2
, 5% (v/v)
CO
2
and 91% (v/v) N
2
, in RPMI 1640
medium with 25 mMHEPES (pH 7.4),
10% (v/v) heat-inactivated human serum,
and sufficient human erythrocytes to achieve
a 2% haematocrit. Parasites were synchro-
nized at the ring stage, by serial treatment
with 5% (v/v) sorbitol (Sigma; Lambros
and Vanderberg, 1979) and studied at 1%
parasitaemia.
Each of compounds 1,2,4and 5was
prepared as a 10-mMstock solution in dimethyl
sulphoxide (DMSO) and diluted as needed
for the individual experiments, with each
test dilution tested in triplicate. The stock
solutions were diluted with HEPES- and
serum-supplemented RPMI 1640 medium
to give (0.2% (v/v) DMSO. Each test
dilution was gently mixed with an equal
volume of parasite culture (showing 1%
parasitaemia and at a 4% haematocrit).
Negative controls contained the same con-
centrations of DMSO but no test com-
pound whereas the cultures used as positive
controls contained 1 mMchloroquine phos-
phate (Sigma) and no test compound. Once
set up, the test cultures were incubated at
37uC for 48 h (representing one cycle of
erythrocytic invasion and intra-erythrocytic
multiplication) before being fixed by repla-
cing the medium with an equal volume of
1% (w/v) formaldehyde in 0.1 Mphosphate-
buffered saline at pH 7.2 (PBS). Aliquots
(50 ml) of each fixed culture were then added
to 5-ml round-bottomed polystyrene tubes,
each of which contained 0.5 ml PBS holding
0.1% (v/v) Triton X-100 and 1.0 nMYOYO
nuclear dye (Molecular Probes, Eugene,
OR). Parasitaemias in the test and control
cultures were then compared using a flow
cytometer (FACSort
TM
; BD, Franklin Lakes,
NJ) to count the nucleated (i.e. parasitised)
erythrocytes in each sample. The counts were
recorded using the CellQuest
TM
software
package (BD), with the test-culture counts
normalized to percentages of the correspond-
ing counts for the positive-control cultures.
ANTIMALARIAL ACTIVITY (K1 STRAIN)
The antimalarial activity of each crude
extract and each of compounds 1,2,4and
5was also assessed quantitatively, in vitro,
using the microculture radio-isotope tech-
nique described by Desjardins et al. (1979),
as modified by Ridley et al. (1996). The
assay uses the uptake of [
3
H]hypoxanthine
by parasites as an indicator of viability.
Continuous in-vitro cultures of the asexual
erythrocytic stages of the pyrimethamine-
and chloroquine-resistant K1 strain of P.
falciparum (Thaithong and Beale, 1981)
were maintained following the methods of
Trager and Jensen (1976). Each extract or
compound was tested after two-fold serial
dilution, at seven concentrations between
20 and 0.31 mg/ml. After incubation of the
ANTIPROTOZOAL ACTIVITIES OF Markhamia 393

parasites with the extract/compound for
48 h at 37uC, [
3
H]hypoxanthine (Amersham
International, Little Chalfont, U.K.) was
added to each well and the incubation was
continued for another 24 h at the same
temperature. Chloroquine (Sigma) was again
used as a positive reference.
LEISHMANICIDAL ACTIVITY
For the in-vitro assays of leishmanicidal
activity, 50 ml of culture medium — a 1 : 1
mixture of SM medium (Cunningham, 1977)
and SDM-79 medium (Brun and
Scho¨nenberger, 1979) at pH 5.4, supplemen-
ted with 10% (v/v) heat-inactivated foetal calf
serum (FCS) — was added to each well of a
96-well microtitre plate (Costar, Cambridge,
MA). Serial dilutions of a crude extract,
compounds 1,2,4or 5or the reference drug
(miltefosine; Zentaris, Frankfurt, Germany)
were prepared in duplicate, in the wells, to
give 50 ml/well and concentrations between
30 and 0.041 mg/ml. Then 10
5
axenically-
grown amastigotes (Bates, 1993) of
Leishmania donovani (MHOM/ET/67/L82)
in 50 ml medium were added to each well,
before the plate was incubated at 37uC,
under a 5%-CO
2
atmosphere, for 72 h. A
12.5% (w/v) aqueous solution of resazurin
was then added, at 10 ml/well, and incuba-
tion continued for a further 2–4 h. The plate
was then read in a microplate fluorometer
(Spectramax Gemini XS; Molecular Devices,
Sunnyvale, CA), using an excitation wave-
length of 536 nm and an emission wavelength
of 588 nm (Raz et al., 1997). Fluorescence
development was measured and expressed as
a percentage of the corresponding positive-
control (miltefosine) value.
ANTITRYPANOSOMAL ACTIVITY
The procedures described by Freiburghaus
et al. (1996) were used to test the in-vitro
activity of each crude extract and isolated
compounds 1,2,4and 5. Working stock
solutions (of 180 mg/ml) were prepared in
the rabbit-serum-containing culture med-
ium described by Baltz et al. (1985) and
dispensed, at 100-ml/well, into the first row
of wells of a 96-well microtitre plate
(Costar). Complete culture medium was
then added to the other wells, so that three-
fold serial dilutions of each extract/com-
pound could be prepared. After the addition
to each well of 2610
3
of the bloodstream
forms of Trypanosoma brucei rhodesiense,
from axenic culture (Baltz et al., 1985), the
concentration of the extract/compound in
each 100-ml culture ranged from 90 to
0.13 mg/ml. After incubation for 72 h in
a humidified atmosphere at 37uC, with
5% (v/v) CO
2
, parasite development was
assessed with resazurin — as for L. donovani
but incubating with the resazurin for 24 h
and using melarsoprol (ArsobalH; Rhoˆne
Poulenc Rorer, Paris), not miltefosine, as
the reference drug.
CYTOTOXICITY
The cytotoxicity of each crude extract and
compounds 1,2,4and 5was assessed using
the L-6 cell line (of rat skeletal-muscle
myoblasts) and the method of Page´ et al.
(1993) as modified by Ahmed et al. (1994).
The rat cells were seeded in 96-well micro-
titre plates (Costar) to give 10
3
cells in
50 ml complete medium [MEM supplemen-
ted with 10% (v/v) heat-inactivated FCS]
in each well. A three-fold serial dilution of
an extract or isolated compound, prepared
in the complete culture medium, was
then added, at 50 ml/well, to give final
concentrations of 90 to 0.13 mg extract or
compound/ml. Plates were then incubated
at 37uC for 72 h in a humidified incubator,
with 5% (v/v) CO
2
. Resazurin was again
added as a viability indicator (Ahmed et al.,
1994), incubating with the resazurin for 2 h
before each plate was ‘read’ on the fluores-
cence scanner. Podophyllotoxin (Polysciences
Inc., Warrington, PA) was used as the
reference drug.
DATA ANALYSIS
Median inhibitory concentrations (IC
50
)
were calculated, for each extract and iso-
394 TANTANGMO ET AL.

lated compound, using the Prism 3.0 soft-
ware package (GraphPad Software, La Jolla,
CA). For this, the assay data were fitted, by
non-linear regression, to the variable-slope
sigmoidal dose–response formula y5100/
(1z10
(logIC50
2x)H
), where H is the Hill
coefficient or slope factor (Singh and
Rosenthal, 2001).
Selectivity indexes (SI) were calculated,
from the results of the assays of antimalarial
and cytotoxic activities, as (IC
50
for the L-6
cells)/(IC
50
for a strain of P. falciparum).
The values given in the Table are mean
results (and S.D.) for either two independent
assays (extracts) or three (pure compounds),
with each assay run in duplicate.
RESULTS AND DISCUSSION
In the present study, three extracts of the
stem bark of M. tomentosa were evaluated
in vitro for their activities against (the
chloroquine-resistant K1 and W2 strains
of) P. falciparum,L. donovani, and T. brucei
rhodesiense — parasites that can cause
human malaria, visceral leishmaniasis and
African trypanosomiasis, respectively. The
ethyl-acetate extract had the best antimalarial
activity, with similarly low IC
50
and similarly
high SI (.50) recorded for the two strains of
P. falciparum that were tested (see Table).
Fractionation of the ethyl-acetate extract
by flash and successive column chromato-
graphy yielded eight compounds. Their
structures were established, by
1
H- and,
13
C-NMR spectroscopy (including one- and
two-dimensional techniques) and mass spec-
trometry, as 2-acetylnaphtho[2,3-b]furan-
4,9-dione (1; Rao and Kingston, 1982),
2-acetyl-6-methoxynaphtho[2,3-b]furan-4,9-
dione (2;Zaniet al., 1991), oleanolic acid (3;
Mahato and Kundu, 1994), pomolic acid (4;
Mahato and Kundu, 1994), 3-acetylpomolic
acid (5; Mahato and Kundu, 1994), tormen-
tic acid (6; Delgado et al., 1989; Mahato and
Kundu, 1994; Li et al., 2009), b-sitosterol
(7; Kovganko et al., 1999) and b-sitosterol-3-
O-b-D-glucopyranose (8;Moghaddamet al.,
2007) (Fig. 1).
Compounds 1,2,4and 5were then
evaluated for their activities against P.
falciparum,L. donovani and T. b. rhodesiense,
to check whether or not each could con-
tribute to the revealed antiprotozoal activity
of the crude ethyl-acetate extract [the anti-
TABLE. In-vitro antiprotozoal and cytotoxic activities of the three crude extracts and isolated compounds
Mean (S.D.) median inhibitory concentration (mg/ml): Selectivity index
Sample
Plasmodium
falciparum
(K1)
P.
falciparum
(W2)
Leishmania
donovani
Trypanosoma
brucei
rhodesiense
L-6
cell
line
P.
falciparum
(K1)
P.
falciparum
(W2)
CRUDE EXTRACT
Hexane .5 4.93 (0.39) .5.5831817
Ethyl-acetate 2.81 (0.06) 1.46 (0.12) .5 1.5 .90 .50 .50
Methanolic .5ND.5.5.90 – –
ISOLATED COMPOUND
10.11 (0.02) 0.16 (0.02) 0.10 (0.02) 0.016 (0.004) 0.1 0.9 0.6
20.44 (0.04) 0.93 (0.04) 0.77 (0.04) 0.05 (0.01) 0.1 0.2 0.1
43.47 (0.90) .5 0.31 (0.03) .5 4.2 1.2 13.7
52.10 (0.7) .5 3.40 (0.7) .5 16.3 7.8 –
REFERENCE DRUG
Chloroquine 0.094 (0.042) 0.039 (0.002)
Miltefosine 0.12 (0.05)
Melarsoprol 0.003 (0.002)
Podophyllotoxin 0.007
ND, Not determined.
ANTIPROTOZOAL ACTIVITIES OF Markhamia 395
 6
6
 7
7
 8
8
1
/
8
100%